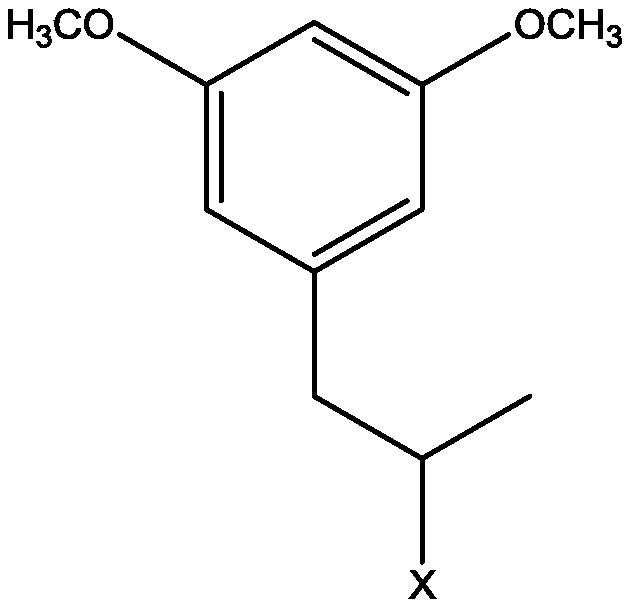
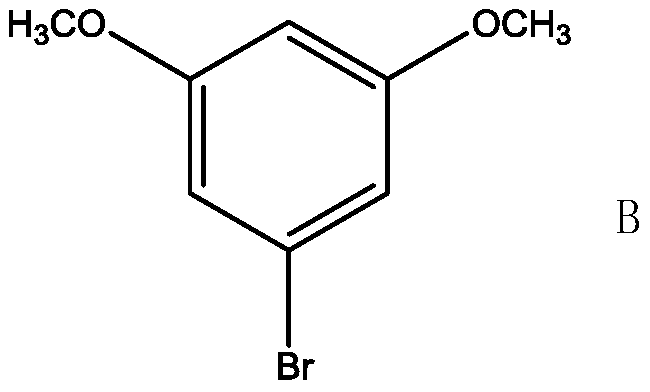
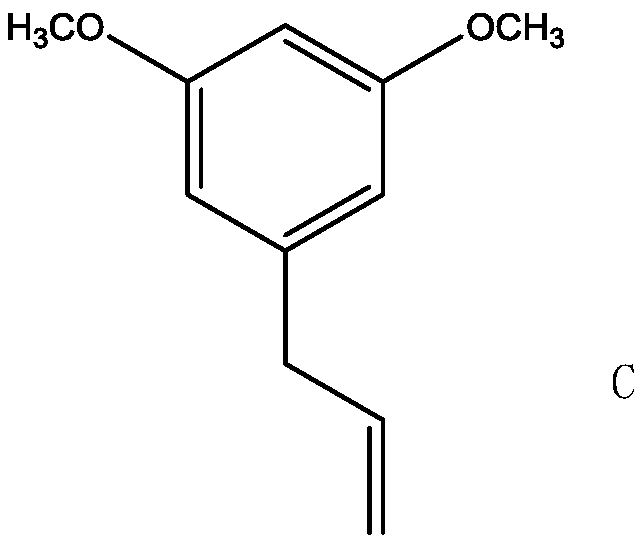
A kind of preparation method of 5-{2-(ethylthio)propyl}-3-hydroxyl-cyclohex-2-enone
A }-3-, ethylthio-based technology, applied in the field of preparation of pesticide intermediates, can solve problems such as increasing the difficulty of tail gas treatment, and achieve the effects of mild reaction process, reduced environmental impact, and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Condensation reaction: Add 21.9g of 5-bromo-m-phenylene dimethyl ether into a 250ml four-necked bottle with a thermometer and a condenser tube {melting point 62-66°C; appearance: off-white solid; NMR: 1 H NMR (CDCl 3 ,400MHz) δ (ppm) data is as follows: 3.82 (s, 6H, CH 3 ), 6.37(t, 1H, Ar-H), 6.81(d, 2H, Ar-H)}, 12.8g bromopropylene, 20.4g triethylamine, 2.2g dppp nickel chloride, 130ml methyl tert-butyl ether , magnetically stirred, heated in an oil bath, kept warm for 4 hours when the temperature rose to 50°C, cooled to room temperature after the reaction, washed the reaction solution with 60ml of 10% hydrochloric acid aqueous solution, washed with water until neutral, and distilled the organic layer under reduced pressure to obtain 17.4g 5-propenyl-m-xylylene ether {melting point 97-100°C; appearance: white solid; NMR: 1 H NMR (CDCl 3 ,400MHz) δ (ppm) data is as follows: 3.23 (d, 2H, CH 2 ), 3.83 (s, 6H, CH 3 ), 4.91 (m, 2H, C=CH 2 ), 5.90 (m, 1H, C=C-H) 6.2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| NMR spectroscopy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com