5,5'-diaryl-3,3'-diamino-4,4'-dimethoxybenzophenone compound and preparation method thereof
A technology of dimethoxybenzophenone and diamino, which is applied in the field of organic synthesis, can solve the problems of limited application, poor solubility of polyimide, and difficult film formation, etc., and achieves convenient operation, reduced production cost, good thermal The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
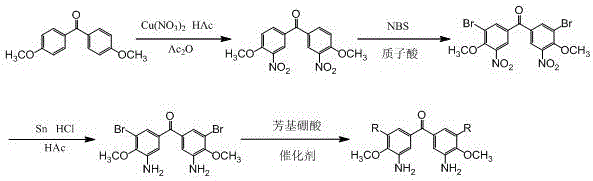
[0029] A kind of preparation method of 5,5'-diaryl-3,3'-diamino-4,4'-dimethoxybenzophenone compound, its preparation method is as follows:
[0030] (I) Synthesis of 3,3'-dinitro-4,4'-dimethoxybenzophenone:
[0031] In a 250mL three-neck flask, add 12g Cu(NO 3 ) 2 ·3H 2 O (50.00mmol), 35mLHAc and 70mL Ac 2 O, stirred at room temperature for 10 min to make Cu(NO 3 ) 2 ·3H 2 O was dissolved, and 4,4'-dimethoxybenzophenone was slowly added to the three-necked flask in batches within 15 minutes, and the temperature was raised to 80°C for 2 hours, followed by TLC spotting, until 4,4'-dimethyl Until the reaction of oxybenzophenone is completed, the reaction solution is poured into distilled water, so that a large amount of white solids are precipitated, filtered under reduced pressure and the filter cake is washed with saturated NaHCO 3 , washed with distilled water, and dried at 80°C to obtain 10.22g of the product 3,3'-dinitro-4,4'-dimethoxybenzophenone, with a yield of 75%....
Embodiment 2
[0039] A kind of preparation method of 5,5'-diaryl-3,3'-diamino-4,4'-dimethoxybenzophenone compound, its preparation method is as follows:
[0040] Steps (I), (II), (III) are the same as in Example 1;
[0041] (IV) Synthesis of 5,5'-bis(4''-α-naphthyl)phenyl-3,3'-diamino-4,4'-dimethoxybenzophenone:
[0042] in N 2 Under protection, add 0.43 g of 5,5'-dibromo-3,3'-diamino-4,4'-dimethoxybenzophenone (1.00 mmol), 0.54g4-(α-naphthyl)phenylboronic acid (2.20mmol), 0.0347gPd(PPh 3 ) 4 (0.02mmol), 0.424g Na 2 CO 3(4.00mmol), 10mL toluene and 5mL distilled water, heated to 110°C for reflux reaction for 6h, a large number of bubbles were generated during the reaction, and tracked by TLC spot plate, after the reaction was complete, cooled to room temperature, filtered to remove insoluble matter, and the filtrate was used A separatory funnel was used to separate the liquid, and the separated aqueous layer was extracted three times with toluene, the extracted organic layers were com...
Embodiment 3
[0044] A kind of preparation method of 5,5'-diaryl-3,3'-diamino-4,4'-dimethoxybenzophenone compound, its preparation method is as follows:
[0045] Steps (I), (II), (III) are the same as in Example 1;
[0046] (IV) Synthesis of 5,5'-bis(4''-9H-carbazolyl)phenyl-3,3'-diamino-4,4'-dimethoxybenzophenone:
[0047] in N 2 Under protection, add 0.30 g of 5,5'-dibromo-3,3'-diamino-4,4'-dimethoxybenzophenone (0.70 mmol), 0.44g4-(9H-carbazolyl)phenylboronic acid (1.54mmol), 0.0243g Pd(PPh 3 ) 4 (0.014mmol), 0.30g Na 2 CO 3 (2.80mmol), 10mL toluene and 5mL distilled water, heated to 100°C for reflux reaction for 6h, a large number of bubbles were generated during the reaction, and tracked by TLC spot plate, after the reaction was complete, cooled to room temperature, filtered to remove insoluble matter, and the filtrate was used A separatory funnel was used to separate the liquid, and the separated aqueous layer was extracted twice with toluene, the extracted organic layers were c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com