Preparation method and application of multicolor halogen perovskite fluorescent material
A fluorescent material and perovskite technology, applied in the direction of luminescent materials, chemical instruments and methods, photovoltaic power generation, etc., can solve the problems of severe phase separation, damage to the morphology of halogen perovskite, and difficult fine control, etc., and meet the conditions Mild, simple equipment, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
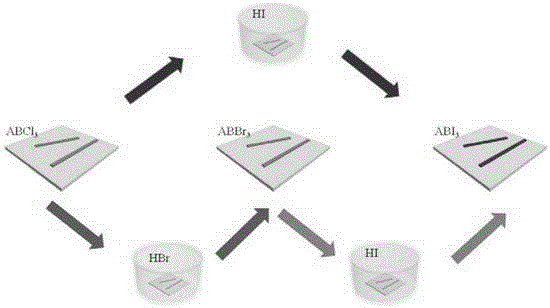
[0030] Preparation of CH with different colors by vapor phase displacement of halogen 3 NH 3 wxya 3 (X=Cl,Br,I) perovskite micro-nanocrystals
[0031] (1) Preparation of CH 3 NH 3 PbCl 3 Perovskite micro-nanocrystal
[0032] Configure 0.01mol / L CH 3 NH 3 Cl and PbCl 2 DMF or DMSO mixed solution, CH 3 NH 3 Cl and PbCl 2 The concentration is 0.01mol / L. Take 200μL of the mixed solution and drop it on the cover glass, place it in the atmosphere of toluene and slowly volatilize it. After the solvent DMF or DMSO volatilizes completely, CH 3 NH 3 PbCl 3 Perovskite micro-nanocrystals.
[0033] (2) CH 3 NH 3 PbCl 3 Conversion of perovskite micro-nanocrystals into CH 3 NH 3 PbBr 3 Perovskite micro-nanocrystal
[0034] The prepared CH 3 NH 3 PbCl 3 The perovskite micro-nano crystals are placed in a petri dish, and the HBr aqueous solution with a mass fraction of HBr of 45% is added dropwise in the petri dish, calculated by the volume of the petri dish, every 10cm 3...
Embodiment 2
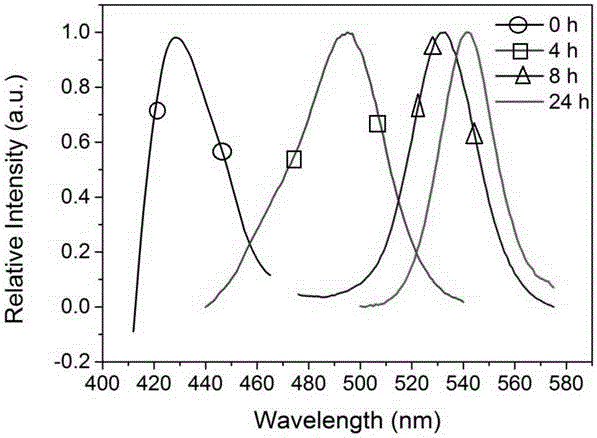
[0039] Preparation of CH with different emission colors by adjusting the time of halogen gas phase replacement 3 NH 3 PbCl n Br 3-n (n=0-3) Perovskites.
[0040] (1) Preparation of CH 3 NH 3 PbCl 3 Perovskite micro-nanocrystal
[0041] Configure 0.01mol / L CH 3 NH 3 Cl and PbCl 2 DMF or DMSO mixed solution, CH 3 NH 3 Cl and PbCl 2 The concentration is 0.01mol / L. Take 200μL of the mixed solution and drop it on the cover glass, place it in the atmosphere of toluene and volatilize slowly. After the solvent volatilizes completely, CH 3 NH 3 PbCl 3 Perovskite micro-nanocrystals.
[0042] (2) HBr gas replaces CH 3 NH 3 PbCl 3 Perovskite micro-nanocrystal
[0043] The prepared CH 3 NH 3 PbCl 3 The perovskite micro-nano crystals are placed in a petri dish, and the HBr aqueous solution with a mass fraction of HBr of 48% is added dropwise in the petri dish, calculated by the volume of the petri dish, every 10cm 3 Add 100 μL dropwise; then add anhydrous calcium chlo...
Embodiment 3
[0045] Preparation of micron-sized CsPbCl 3 -CsPbBr 3 two-color array
[0046] (1) Preparation of CsPbCl 3 Perovskite micro-nanocrystal array
[0047] Preparation of CsPbCl by Template Confinement Method 3 Perovskite micro-nano crystal array;
[0048] (2) part of the CsPbCl 3 Perovskite replaced by HBr gas to CsPbBr 3 perovskite
[0049] CsPbCl 3 A part of the perovskite micro-nanocrystal array is pasted and protected with scotch tape, and placed together in a petri dish, and an HBr aqueous solution with a mass fraction of HBr of 46% is added dropwise to the petri dish, calculated by the volume of the petri dish, every 10cm 3 Add 100 μL dropwise; then add anhydrous calcium chloride particles into the petri dish, the amount of anhydrous calcium chloride particles added is 4 grams per 100 μL of HBr aqueous solution; finally seal the opening of the petri dish with a parafilm, so that CH 3 NH 3 PbCl 3 The perovskite micro-nano crystals are in the airtight HBr atmosphere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com