Method and application of heterologous high expression of dypb active protein
An active protein, high expression technology, applied in the field of heterologous high expression of DypB active protein, can solve problems such as increasing product cost, reducing production efficiency, increasing production links, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Construction of BL21-AL expression host
[0046] (1) Construction of recombinant vector pET28a-hemAL-cm
[0047] The hemA gene and hemL gene of Bacillus subtilis were retrieved through NCBI, the codons were optimized, and the rare codons expressed by E.coli were removed through synonymous mutation, and the hemA gene and nucleotide sequence with the nucleotide sequence of Seq ID No.1 were obtained It is the hemL gene of Seq ID No.2, two genes are connected in series, (G 4 S)x2 is linker, tac promoter to start expression, 36bp homology arm at the 3rd end of thrA gene whose nucleotide sequence is Seq ID No.11 is added to the hemL end, and finally enzyme cutting sites (BglⅡ) and (EcoRI) are added at both ends It was synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd. The synthetic sequence was inserted into pET28a plasmid via (BglⅡ) and (EcoRI) to construct pET28a-hemAL.
[0048] Using the plasmid pkd3 template, using the nucleotide sequence as the primer 1...
Embodiment 2
[0051] Example 2 Construction of pCspA-dypB expression vector
[0052] The dypB gene is derived from Rhodococcus jostii RHA1. The dypB gene sequence of Rhodococcus jostii RHA1 was retrieved through NCBI, the codons were optimized, and enzyme cutting sites (NdeⅠ) and (HindⅢ) were added at both ends, and delivered to Suzhou Jinweizhi Biotechnology Co., Ltd. synthesis. The synthetic sequence was inserted into the pET28a vector through the restriction site mentioned above to construct pET28-dypB.
[0053] Using E.coli as a template, using primer 5 with the nucleotide sequence of Seq ID No.5 and primer 6 with the nucleotide sequence of Seq ID No.6, PCR amplifies the cspA gene promoter, and adds enzyme cutting sites at both ends Point XbaI and NcoI and insert this restriction site into pET28-dypB to construct pCspA-dypB.
Embodiment 3
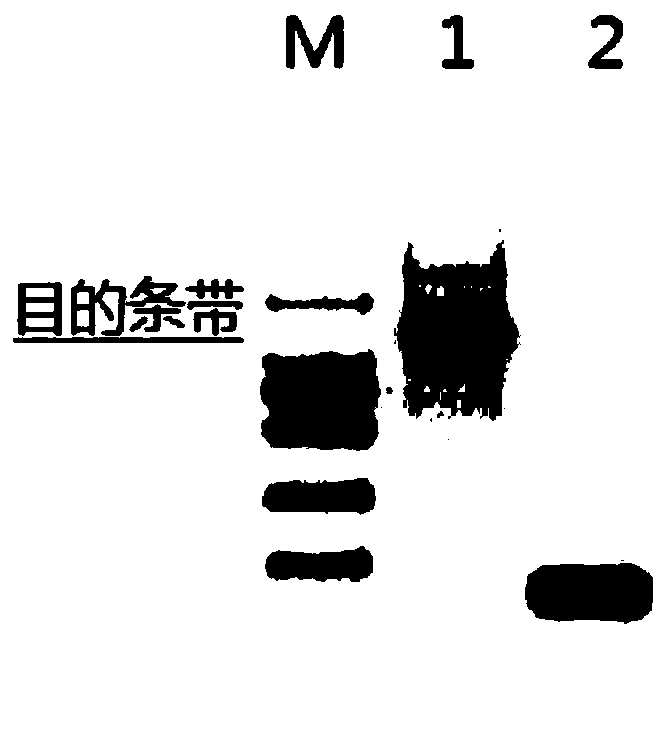
[0054] Example 3 Expression and purification of dypB protein
[0055] The plasmid pCspA-dypB was introduced into BL21-AL and BL21(DE3) expression hosts respectively, and positive single colonies were cultured at 37°C until OD 600 When = 0.8, add IPTG to a final concentration of 0.5mM, and induce at 16°C for 20 hours; collect the bacteria and resuspend them with 1 / 4 of the bacterial volume in buffer (20mM Tris-HCl, 5% glycerol), sonicate, and collect Supernatant; purified by nickel affinity chromatography: 20mM Tris-HCl, 500mM NaCl, 5mM imidazole equilibrated, 20mM Tris-HCl, 500mM NaCl, 60mM imidazole eluted impurity protein, 20mM Tris-HCl, 500mM NaCl, 500mM imidazole eluted target protein. The eluate was replaced with 20mM Tris-HCl, 5% glycerol dialyzed for 12 hours, protein quantification was performed with BCA protein quantification kit, and the concentration was adjusted to 1 mg / ml and stored at -80.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com