Quality Control Method of Shenrong Yijing Tablets
A quality control method, the technology of Shenrong Yijing Tablets, applied in the field of medicine, can solve the problems of white blood cell and hemoglobin reduction, and achieve the effect of safe and effective medicine, controllable quality and good quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1, the identification of Radix Astragali
[0036] Take 3g of Shenrong Yijing Tablets, remove the coating according to the conventional method, grind it into powder, add 20ml of methanol to the powder, heat and reflux for 1h, filter, evaporate the filtrate to dryness, add 30ml of water to dissolve the residue, and then saturate it with n-butanol water The solution was extracted twice, 20ml each time, the n-butanol extract was combined, and the n-butanol extract was washed twice with water, 20ml each time, the water was discarded, the washed n-butanol solution was evaporated to dryness, and the residue was added methanol 0.5 ml dissolved, as the test solution.
[0037]Take astragaloside IV, add methanol to make a reference substance solution containing 1mg per 1ml.
[0038] Methanol was used as a negative control solution.
[0039] According to the thin-layer chromatography test, draw 2 μl of each of the above three solutions, and place them on the same silic...
Embodiment 2
[0041] Embodiment 2, the identification of ginseng
[0042] Take 5g of Shenrong Yijing Tablets, remove the coating according to the conventional method, grind it into powder, put it in a stoppered Erlenmeyer flask, add 50ml of methanol, heat and reflux for 2h, let it cool, filter, measure 25ml of the filtrate and evaporate to dryness, Add 30ml of water to the residue to dissolve, transfer to a separatory funnel, shake and extract with chloroform twice, 30ml each time, discard the chloroform, then shake and extract with n-butanol water-saturated solution 5 times, 20ml each time , combined n-butanol extract, washed twice with ammonia test solution, 30ml each time, and then washed with n-butanol water saturated solution 30ml, took n-butanol solution and evaporated to dryness, the residue was dissolved in 1ml methanol, as the test solution .
[0043] Take ginsenoside Rg1 and Re and add methanol to make a mixed reference solution containing 2 mg per 1 ml.
[0044] Methanol was us...
Embodiment 3
[0047] Embodiment 3, ginsenoside Rg 1 and ginsenoside Re content determination
[0048] (1) Method of content determination
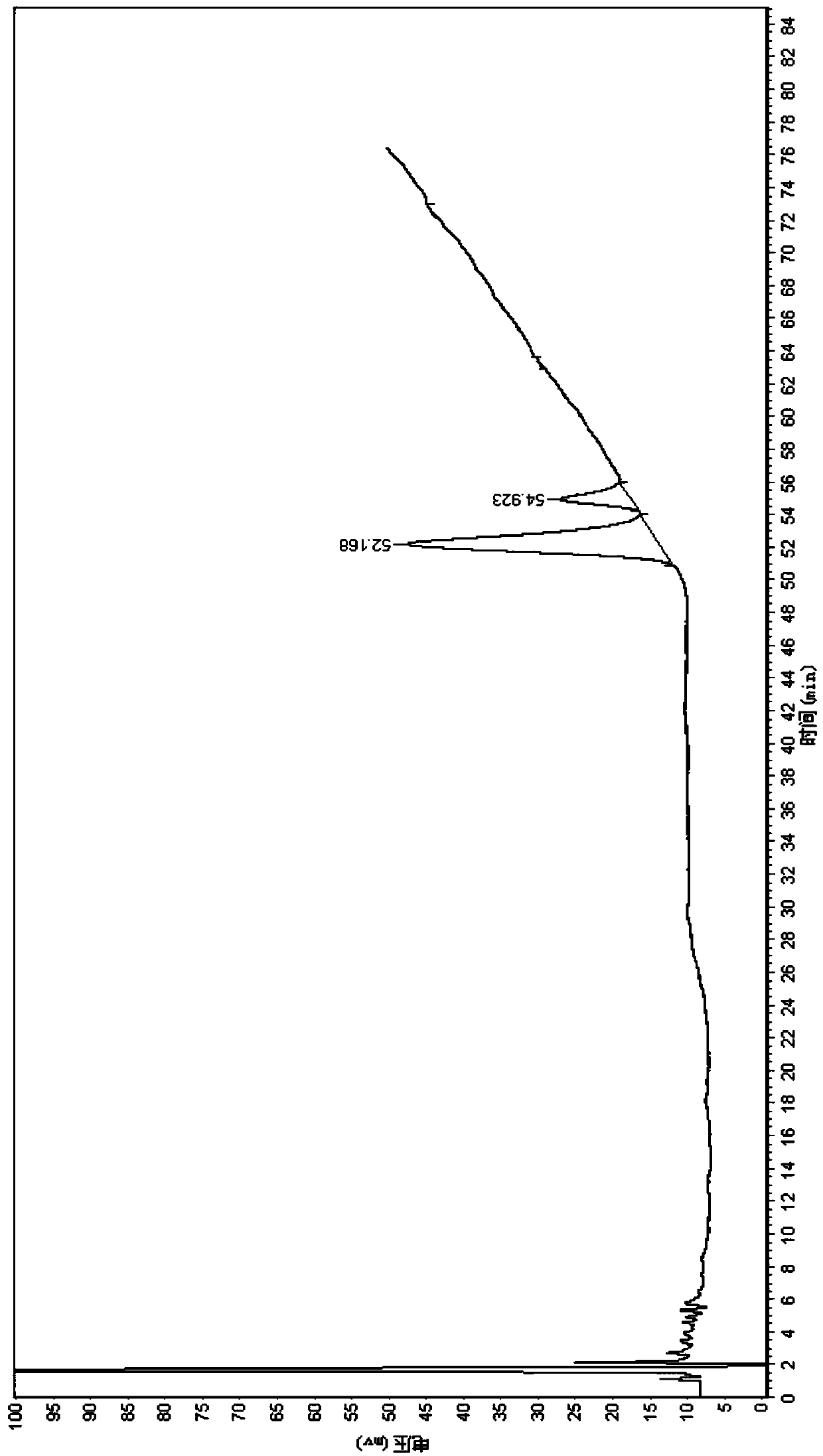
[0049] Ginseng is the main medicinal taste in Shenrong Yijing Tablets. This method adopts high performance liquid phase method to treat ginseng with ginsenoside Rg 1 and ginsenoside Re as indicators for content determination.
[0050] Chromatographic conditions and system suitability test: Octadecylsilane bonded silica gel was used as filler; acetonitrile was used as mobile phase A, water was used as mobile phase B, and gradient elution was performed; column temperature was 40°C; flow rate was 1.0ml / min, the detection wavelength is 203nm, and the number of theoretical plates is ≥6000 based on the peak of ginsenoside Rg1.
[0051] The elution gradient is:
[0052]
[0053]
[0054] Preparation of reference solution: Accurately weigh 0.2 mg ginsenoside Rg 1 Reference substance, ginsenoside Re reference substance, add 1ml of methanol, shake wel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com