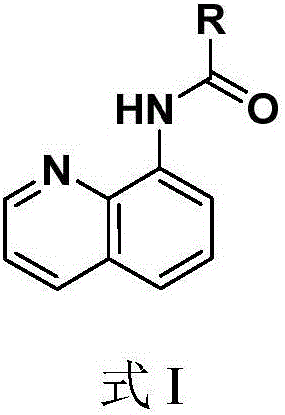
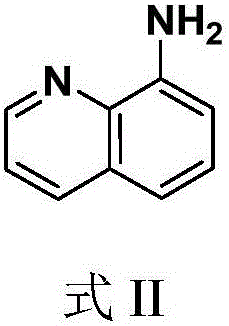
Quinoline amide compounds and preparation method and application thereof
A compound, quinoline technology, which is applied in the field of quinoline amide compounds and their preparation, can solve the problems of brassinolide synthesis cost, easy metabolism inactivation, and many metabolic sites, so as to improve lodging resistance, The effect of easy preparation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
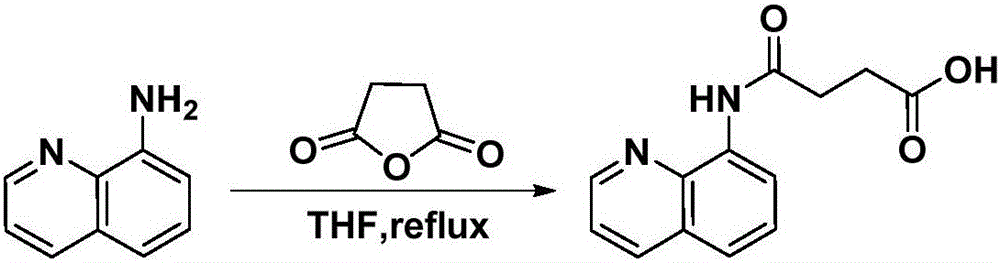
[0056] Embodiment 1, the preparation of compound 1a:
[0057]
[0058] In a 100mL round-bottomed flask, add 8-aminoquinoline (10mmol) and succinic anhydride (12mmol), stir and dissolve 15mL tetrahydrofuran (solvent A), heat up and reflux for 2 hours for acylation reaction, cool down and cool down to precipitate a solid, filter A solid was obtained and purified by recrystallization from ethanol. The specific method of recrystallization is: add 3 mL of ethanol dropwise to 1 g of the primary product to be recrystallized, heat to reflux until the primary product is completely dissolved, and continue to reflux for 10 minutes, then slowly cool down to precipitate a solid, and filter with suction to obtain a pure product.
Embodiment 2
[0059] Embodiment 2, the preparation of compound 1b:
[0060]
[0061] In a 100mL round-bottomed flask, add 8-aminoquinoline (10mmol), succinic anhydride (12mmol), 15mL tetrahydrofuran and stir to dissolve, reflux for 3 hours for acylation reaction, cool down to precipitate a solid, filter to obtain a solid, and recrystallize from ethanol The intermediate product was purified. Take the intermediate product (10mmol), dissolve it in 3ml of methanol, cool it to 0°C in an ice-salt bath, add 5ml of THF solution that has been prepared to obtain thionyl chloride dropwise at a constant temperature, and complete the dropwise addition in 30 minutes; continue to react for 5 hours, and pour the reaction mixture into 150mL In ice water, extracted with ethyl acetate (3x100mL), washed with saturated brine (3x100mL), anhydrous Na 2 SO 4 After drying, concentration by filtration, the crude product was purified by recrystallization from ethanol or ethyl acetate. The specific method of rec...
Embodiment 3
[0062] Embodiment 3, the preparation of compound 1d:
[0063] Compound 1d was prepared as follows:
[0064]
[0065] In a 100mL round bottom flask, add 8-aminoquinoline (10mmol), 3mL (20mmol) triethylamine, 15ml tetrahydrofuran and stir to dissolve, then add TBTU (3.531g, 11mmol) and Boc-amino acid (10mmol). The reaction mixture was stirred at room temperature for acylation reaction for 12 h, and the progress of the reaction was detected by TLC. After the reaction, concentrate and dissolve with dichloromethane (50mL), adjust pH to 10 with 1M NaOH, after the system is separated, take the aqueous phase, acidify to pH=3 with 1M HCl, extract with ethyl acetate (3×100mL), wash with saturated brine ( 3x100mL), anhydrous Na 2 SO 4 After drying, concentration by filtration, the crude product was purified by recrystallization from ethanol. The specific method of recrystallization is: add 3 mL of ethanol dropwise to 1 g of the primary product to be recrystallized, heat to reflux ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com