Novel hydrophilic-lipophilic gel membrane and preparation method thereof
A gel film and amphiphilic technology, applied in the field of amphiphilic gel materials, can solve problems such as reducing the ability of the skin and the outside world, destroying hair follicles, and affecting the activity of skin cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
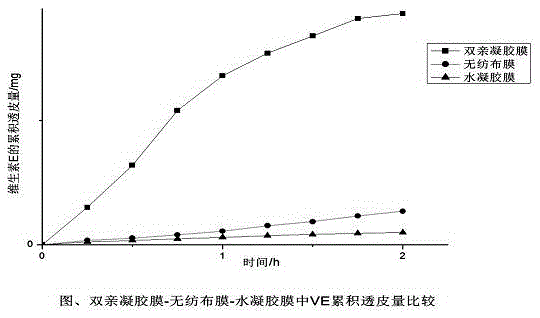
Image
Examples
Embodiment 1
[0061] In this experiment, the amphiphilic gel material was prepared by using styrene-isoprene-styrene block copolymer with a styrene content of 15% as the skeleton material, liquid paraffin as the softener, and hydrogenated rosin glyceride as the tackifier. details as follows:
[0062] Amphiphilic Gel Matrix Formulation:
[0063] component name Styrene and Isoprene Block Copolymer Hydrogenated Rosin Glycerides liquid paraffin hyaluronic acid Sodium polyacrylate Other functional additives ContentWt / % 24.3 21.2 41.5 2 5.4 5.6
[0064] Other functional additives include: inorganic fillers such as calcium carbonate, talcum powder, zinc oxide, etc. to adjust the shape of the amphiphilic gel material.
[0065] The amphiphilic gel matrix preparation method is as follows:
[0066] 1) Add "styrene-isoprene-styrene block copolymer" and liquid paraffin into a reaction kettle with a stirring paddle, heat and stir at a certain temperature until "styre...
Embodiment 2
[0072] In this experiment, a combination of styrene-isoprene-styrene block copolymer and styrene-isoprene diblock copolymer with a styrene content of 15% was used as the skeleton material, and low molecular weight polyisobutylene with a molecular weight of 400 Amphiphilic gel materials were prepared as softeners, hydrogenated rosin glycerides and polyisobutylenes of different molecular weights as tackifiers, as follows:
[0073] Amphiphilic gel matrix material formulation:
[0074] component name Styrene-isoprene-styrene block copolymers and styrene-isoprene diblock copolymers Hydrogenated Rosin Glycerides Medium Molecular Weight Polyisobutylene Polyisobutylene Mn=400 Sodium hyaluronate Sodium carboxymethyl cellulose Sodium polyacrylate Other functional additives ContentWt / % 18.3 12.3 5.9 46.7 2.5 2.7 6.7 4.9
[0075] Other functional additives include: inorganic fillers such as calcium carbonate, talcum powder, zinc oxide, etc. to adj...
Embodiment 3
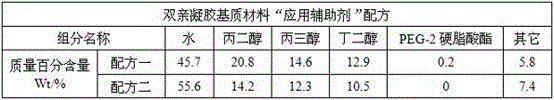
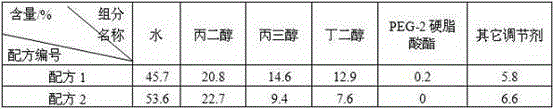
[0083] In this experiment, the amphiphilic gel material "application aid" was prepared, as follows:
[0084]
[0085] The requirement for the preparation of the "application aid" of the amphiphilic gel material is that the viscosity of the liquid should not be too large or too small, otherwise it will affect the infiltration effect with the skin and the loosening effect on the stratum corneum, making the nutritional ingredients of the drug The skin penetration effect is greatly affected, and at the same time, too sticky will make the skin's comfort worse.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com