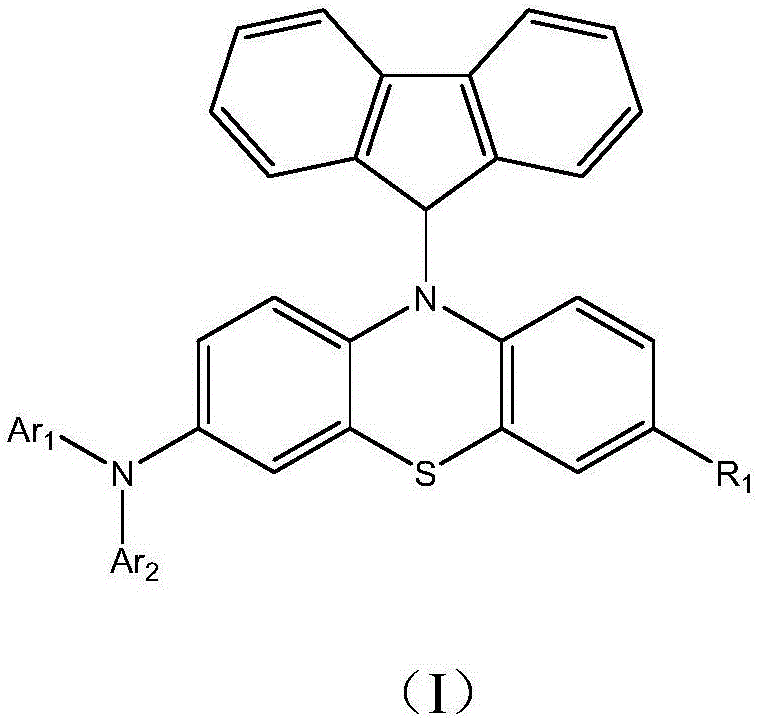
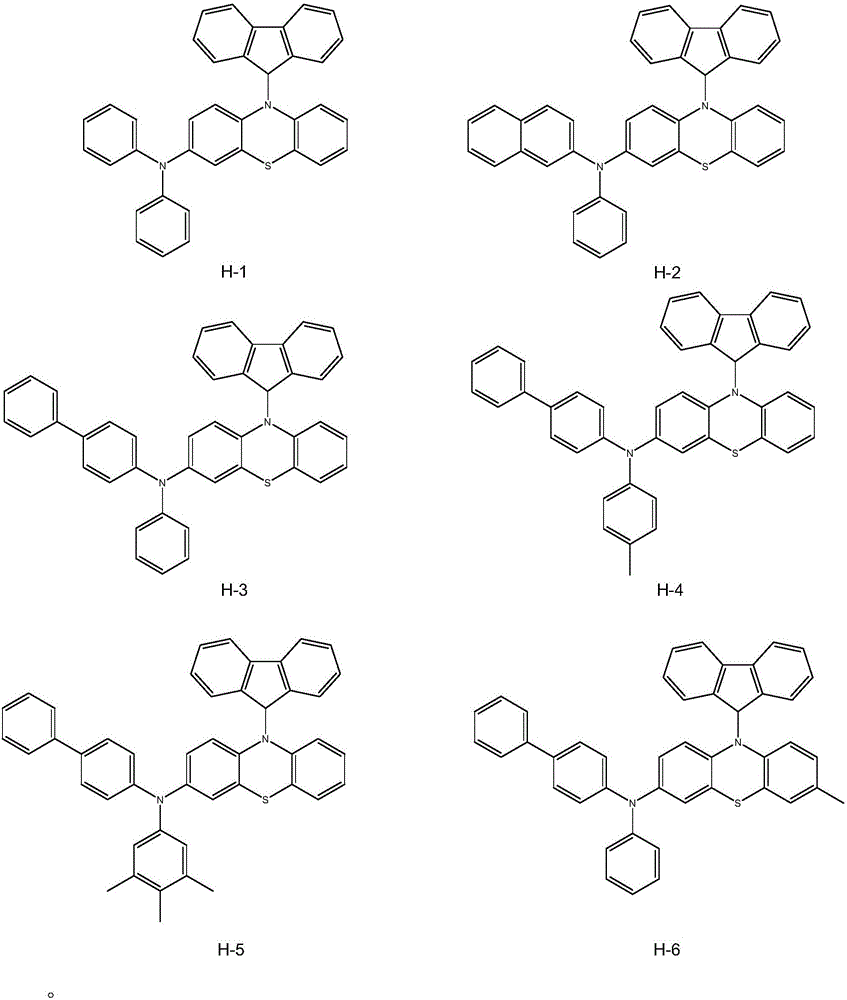
Heterocyclic compound and organic light emitting device thereby
A technology of organic light-emitting devices and heterocyclic compounds, applied in the field of organic light-emitting devices, can solve problems such as low luminous brightness, high driving voltage, and efficiency to be improved, and achieve high luminous brightness, low switching voltage, and improved luminous characteristics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The preparation method of the heterocyclic compound represented by the formula (I) of the present invention first utilizes the reaction of brominated aromatic compound and aromatic amine derivatives to prepare aromatic diamine series compounds. The bromide of the phenothiazine derivative is then prepared, and the phenothiazine-derived diamine compound is prepared through a substitution reaction. Finally, the compound of formula (I) is prepared by the substitution reaction of diamine compound derived from bromofluorene and phenothiazine, and the reaction process is as follows:
[0041]
[0042] The present invention has no special requirements on the reaction conditions of the above-mentioned various reactions, and the conventional conditions of this type of reaction well known to those skilled in the art can be used. The present invention has no special limitation on the sources of the raw materials used in the above-mentioned various reactions, which can be commerci...
Embodiment 1
[0046] Embodiment 1: the synthesis of compound H-1
[0047] 1. Under nitrogen protection, mix 80mmol of 1-bromobenzene, 90mmol of aniline, 3.2mmol of palladium acetate, 100mmol of cesium carbonate and 4.2mmol of BINAP in a reaction flask in proportion, and add 200ml of dioxane to dissolve the reactant. Heated to reflux and reacted for 12 hours. After cooling the reaction solution to room temperature, it was filtered, washed with dichloromethane, and the organic layer was washed twice with 2M hydrochloric acid and then washed with water. After the solvent was evaporated, the product was separated and purified by column chromatography.
[0048]
[0049] 2. Under the protection of nitrogen, add the p-phenylenediamine and phenothiazine derivatives prepared in step 1 to toluene according to the molar ratio of 1:1, and add potassium tert-butylate and palladium acetate as catalysts at the same time, and heat the reactant to 80 °C for 3 hours and then cooled to room temperature. ...
Embodiment 2
[0053] Embodiment 2: the synthesis of compound H-2
[0054] 1. Under the protection of nitrogen, mix 80mmol of 1-bromonaphthalene, 90mmol of aniline, 3.2mmol of palladium acetate, 100mmol of cesium carbonate and 4.2mmol of BINAP in the reaction flask in proportion, and add 200ml of dioxane to dissolve the reactant. Heated to reflux and reacted for 12 hours. After cooling the reaction solution to room temperature, it was filtered, washed with dichloromethane, and the organic layer was washed twice with 2M hydrochloric acid and then washed with water. After the solvent was evaporated, the product was separated and purified by column chromatography.
[0055]
[0056] 2. Under the protection of nitrogen, add the aromatic diamine and phenothiazine derivatives prepared in step 1 to toluene at a molar ratio of 1:1, and at the same time add potassium tert-butylate and palladium acetate as catalysts, and heat the reactant to 80°C After 3 hours of reaction, the temperature was lowe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com