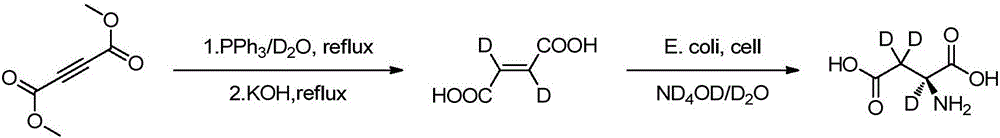
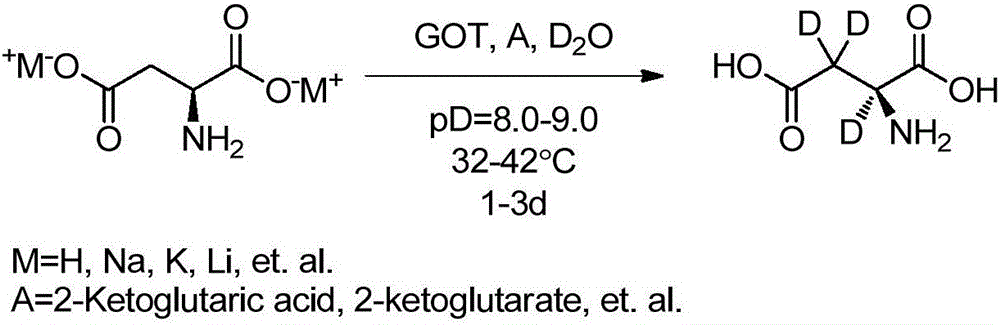
Preparation method of L-aspartic acid (2, 3, 3-D3) by enzymatic catalyst
A technology for aspartic acid and catalytic preparation, applied in directions such as fermentation, can solve the problems of high synthesis cost of chiral synthons, low comprehensive yield, cumbersome process, etc., and achieves saving solvent and synthesis cost, high deuterium abundance, The effect of simplifying purification steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Into a 100ml three-neck flask connected with argon protective gas and a thermometer, add 300mg L-aspartic acid and 10mg α-ketoglutaric acid in sequence, replace the oil pump three times, then add 10mL deuterium water, and then add 2-5 drops of deuterium Dissolve the solid with sodium oxide solution (10%), then adjust the pD value to 8.0-9.0 with sodium deuterium oxide solution, and finally add 20-50 U of aspartate aminotransferase (GOT), keep it sealed and keep warm at 32-36 ° C for reaction 3 - 5 days, LC-MS to monitor the reaction. After the reaction is over, first remove a small amount of solids and enzymes by filtering with a microporous membrane, and then recover deuterium water by distillation under reduced pressure, about 70-80% of deuterium water is evaporated, then adjust the pH value of the solution to 2.5-3.0 with dilute hydrochloric acid, and let it stand Refrigerated and crystallized (0-10°C), a white solid precipitated out. Filtration, the filter cake was...
Embodiment 2
[0030] Into a 100ml three-necked flask connected with nitrogen protection gas and a thermometer, sequentially add 500mg of L-aspartic acid disodium salt and 10mg of α-ketoglutarate disodium salt, replace the oil pump three times, and then add 15mL of deuterium water to dissolve the solid , and then use deuterated sodium oxide solution to adjust its pD value to 8.0-9.0, and finally add 20-50 U of aspartate aminotransferase (GOT), keep it sealed at 32-39 ° C for 1-3 days, and monitor the reaction by LC-MS. After the reaction is over, first remove a small amount of solids and enzymes by filtering with a microporous membrane, and then recover deuterium water by distillation under reduced pressure, about 70-80% of deuterium water is evaporated, then adjust the pH value of the solution to 2.5-3.0 with dilute hydrochloric acid, and let it stand Refrigerated and crystallized (0-10°C), a white solid precipitated out. Filtration, the filter cake was washed with ice water, and dried to o...
Embodiment 3
[0032] Into a 100ml three-neck flask connected with argon protective gas and a thermometer, add 300mg L-aspartic acid, 10mg α-ketoglutarate, and 167mg lithium carbonate in sequence, replace the oil pump three times, and then add 10mL deuterium water to dissolve the solid. Gas will be produced during this period, and then adjust its pD value to 8.0-9.0 with lithium carbonate deuterium aqueous solution, and finally add 20-50U of aspartate aminotransferase (GOT), keep it sealed and keep it warm at 34-40°C for 2-4 days, LC- MS monitored the reaction. After the reaction is over, first use a microporous membrane filter to remove a small amount of solids and enzymes, then distill under reduced pressure to recover deuterium water, about 70-80% of deuterium water is evaporated, then adjust the pH value of the solution to 2.5-3.0 with dilute hydrochloric acid, add 10 Doubling the volume of absolute ethanol, standing in cold storage for crystallization (0-10°C), a white solid precipitate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com