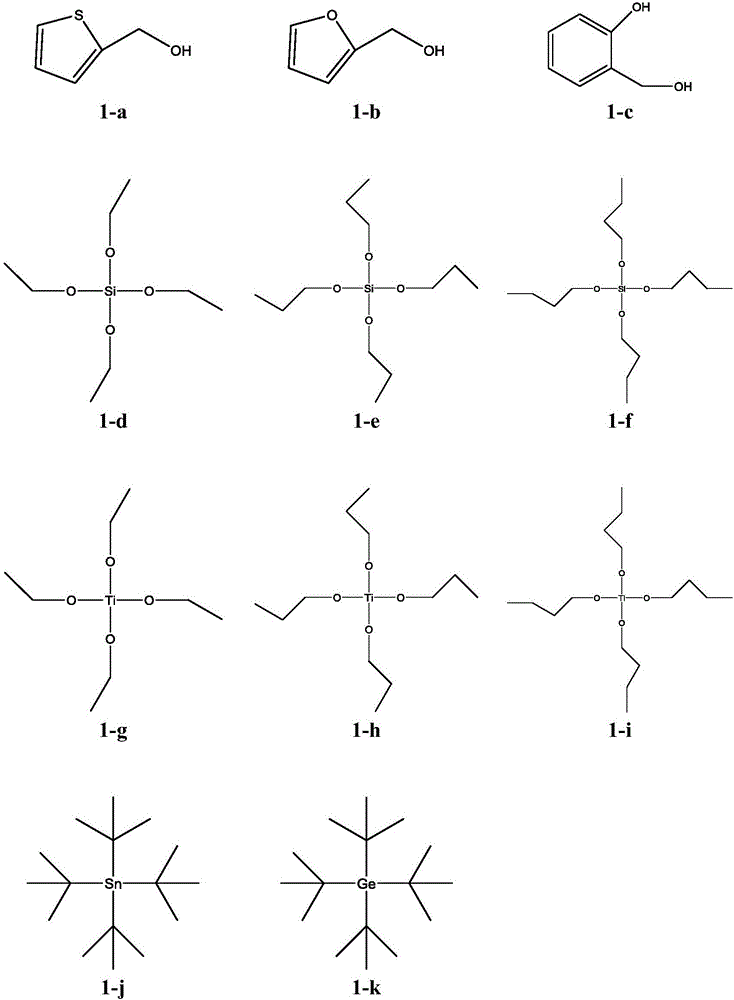
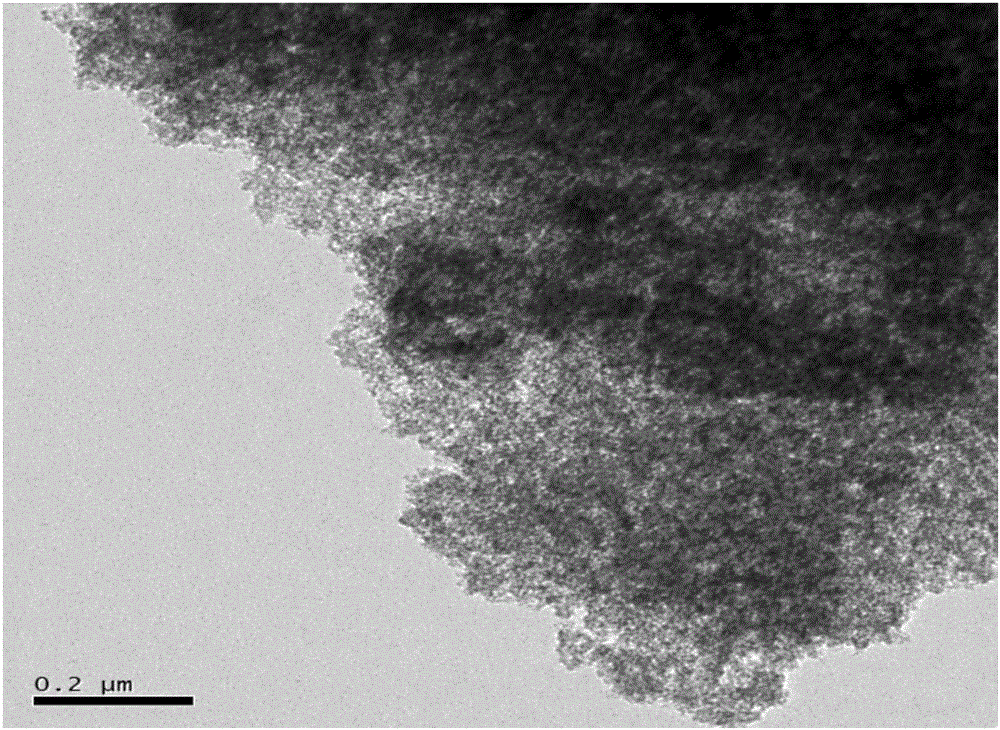
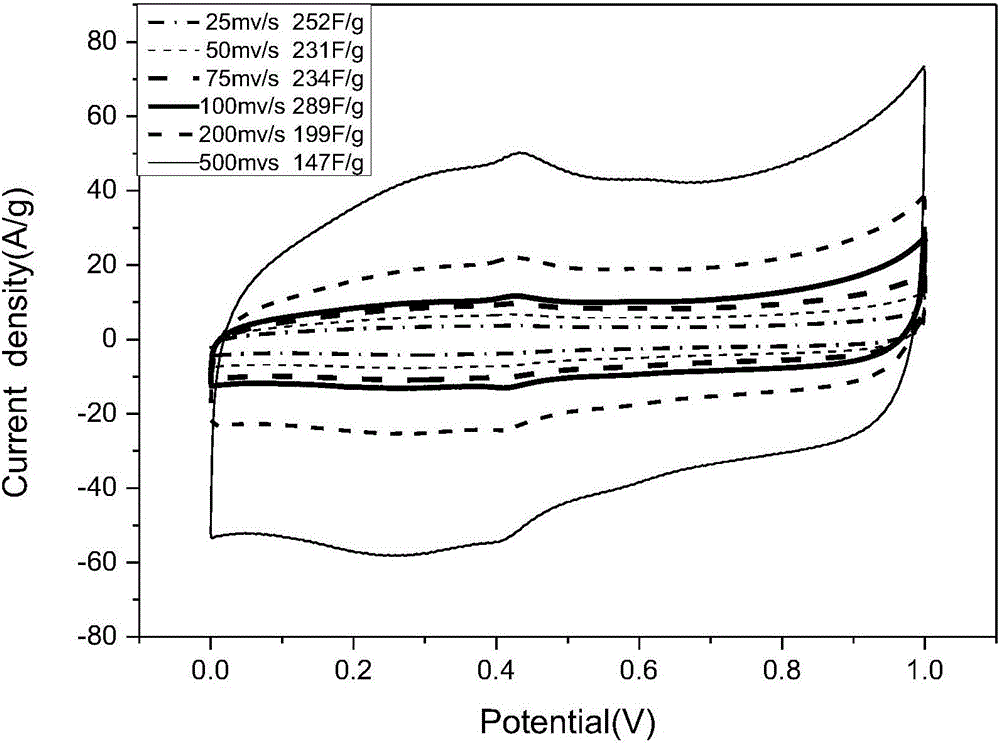
Method for preparing electrode material through synchronous polymerization method
A technology for preparing electrodes and polymerization methods, which is applied in battery electrodes, carbon preparation/purification, circuits, etc., can solve the problems of poor rate cycle performance, poor conductivity, and high cost, and achieve good rate performance, good cycle performance, and specific electric capacity The effect of large capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of sulfur-doped carbon materials by simultaneous polymerization, according to the following steps:
[0028] ① Measure highly purified 2-thiophene methanol 10.0g, highly purified tetraethyl silicate 4.90mL (the molar ratio of the hydroxyl group in 2-thiophene methanol to the ester group in tetraethyl silicate is 1:1), Then put it into a round bottom flask and stir well.
[0029] ②Measure 442μL ([M] / [I]=5) of trifluoroacetic acid and dissolve it in 20.0mL of dichloromethane, stir well to make it evenly mixed, and slowly add the solution dropwise to a container containing 2- In the round bottom flask of the mixture of thiophene methanol and tetraethyl silicate, add it dropwise in about half an hour.
[0030] ③Set up the condensing reflux device, heat and stir at 90°C for 2.5h to obtain the brown product A (mixed liquid), then take out the product A, let it stand at room temperature, add a large amount of dichloromethane for extraction after cooling, and take t...
Embodiment 2
[0036] Preparation of sulfur-doped carbon materials by simultaneous polymerization, according to the following steps:
[0037] ① Measure highly purified 2-thiophene methanol 10.0g, highly purified tetrapropyl silicate 5.05mL (the molar ratio of the hydroxyl group in 2-thiophene methanol to the ester group in tetrapropyl silicate is 1:1), Put into a round bottom flask and stir well.
[0038]② Measure 501.0 μL of trifluoromethanesulfonic anhydride ([M] / [I]=5) and dissolve it in 20.0 mL of dichloromethane, stir well to make it evenly mixed, and slowly add the solution dropwise to In the round bottom flask containing the mixture of 2-thiophene methanol and tetrapropyl silicate, add it dropwise in about half an hour.
[0039] ③Set up the condensing reflux device, and heat and stir the monomer added with the catalyst at 80°C for 3 hours to obtain the brown product A (mixed liquid), then take out the product A, let it stand at room temperature, and add a large amount of dichlorometh...
Embodiment 3
[0043] Preparation of sulfur-doped carbon materials by simultaneous polymerization, according to the following steps:
[0044] ① Measure highly purified 2-thiophene methanol 10.0g, highly purified tetrabutyl silicate 5.15mL (the molar ratio of the hydroxyl group in 2-thiophene methanol to the ester group in tetrabutyl silicate is 1:1), Put into a round bottom flask and stir well.
[0045] ② Measure 501.0 μL of trifluoromethanesulfonic anhydride ([M] / [I]=5) and dissolve it in 20.0 mL of dichloromethane, stir well to make it evenly mixed, and slowly add the solution dropwise to In the round bottom flask containing the mixture of 2-thiophene methanol and tetrabutyl silicate, add it dropwise in about half an hour.
[0046] ③Set up the condensing reflux device, and heat and stir the monomer added with the catalyst at 80°C for 3 hours to obtain the brown product A (mixed liquid), then take out the product A, let it stand at room temperature, and add a large amount of dichloromethan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com