A kind of preparation method of venlafaxine hydrochloride intermediate
A technology of venlafaxine hydrochloride and intermediates, which is applied in the field of medicine, can solve the problems of dangerous reaction, low yield, difficult post-processing, etc., and achieve the effects of easy industrial production, safe and controllable operation, and enhanced catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
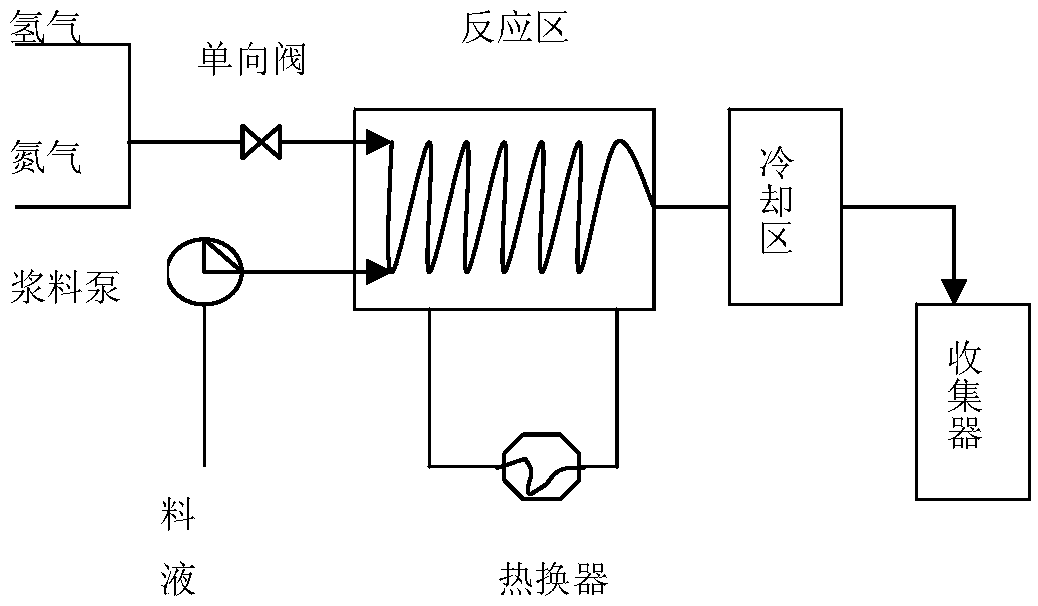
[0032] 1) Preparation of feed solution: Dissolve 100g of 1-cyano-[(4-methoxyphenyl)methyl]cyclohexanol in 200ml of toluene, stir to dissolve, and obtain a feed solution for later use.
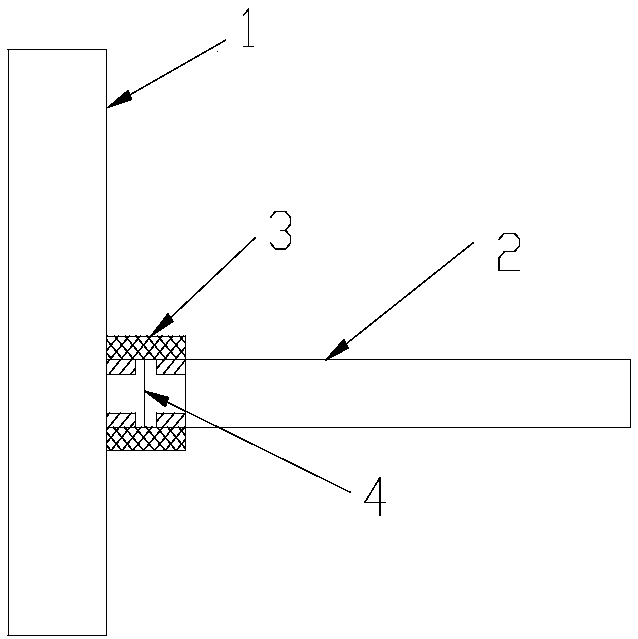
[0033] 2) Microreactor reaction: Install the Raney nickel screen at the pipe connection, use nitrogen to fully replace the air in the reaction zone of the microreactor, then preheat the reaction zone of the microreactor to an internal temperature of 30°C, and then pass Enter the hydrogen gas to control the pressure of 0.2-0.3MPa, and inject the feed liquid in step 1) into the reaction zone of the microreactor through the slurry pump to control the temperature at 30°C for 3-8s; Water cooling) after cooling, collect reaction solution;
[0034] 3) Generation of 1-[2-amino-1-(4-methoxyphenyl)ethyl]cyclohexanol sulfate: the reaction solution was cooled to room temperature, filtered to remove insoluble matter, and slowly added dropwise to the filtrate 11.5ml Concentrated sulfuric acid (18mol / L), slo...
Embodiment 2
[0036] 1) Preparation of slurry: Dissolve 100g of 1-cyano-[(4-methoxyphenyl)methyl]cyclohexanol in 200ml of ethyl acetate, stir to dissolve, and obtain a feed solution for later use;
[0037]2) Microreactor reaction: Install the Raney nickel screen at the pipe connection, use nitrogen to fully replace the air in the reaction zone of the microreactor, preheat the microreactor to an internal temperature of 40°C, and inject hydrogen to control the reaction. The pressure is 0.3-0.4MPa, the feed liquid is pumped into the microreactor by the slurry pump, and the temperature is controlled in the reaction area at 40°C for 1-5s; after the reaction is completed, it is cooled by the cooling area (the cooling area is cooled by circulating water), Collect the reaction solution;
[0038] 3) Generation of 1-[2-amino-1-(4-methoxyphenyl)ethyl]cyclohexanol sulfate: the reaction solution was cooled to room temperature, filtered to remove insoluble matter, and slowly added dropwise to the filtrat...
Embodiment 5
[0042] Preparation of venlafaxine hydrochloride
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com