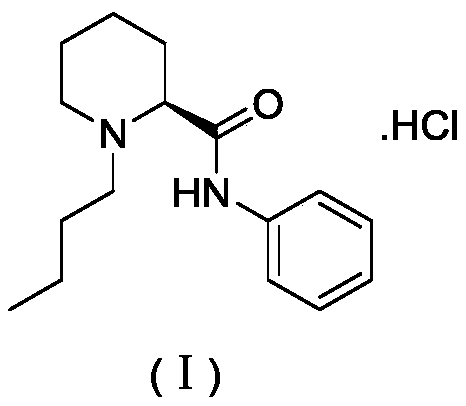
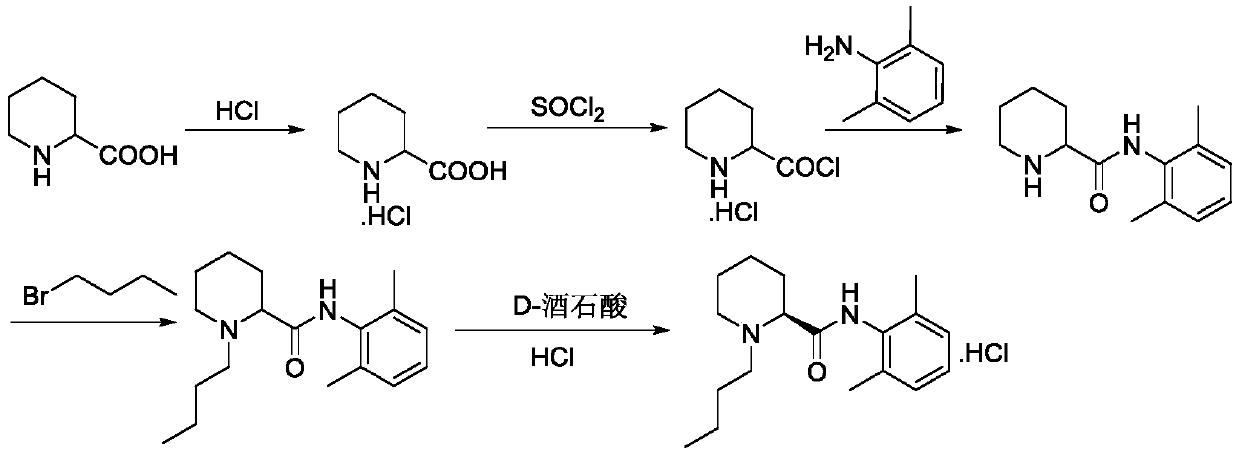
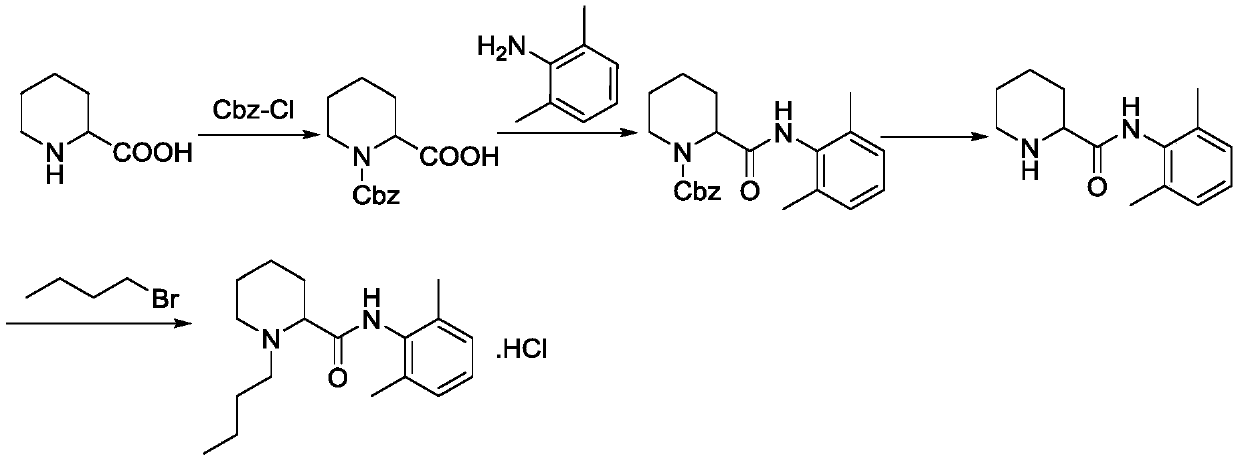
A kind of preparation method of levobupivacaine hydrochloride
A technology of levobupivacaine hydrochloride and carboxylic acid, which is applied in the field of preparation of levobupivacaine hydrochloride, can solve the problems of harsh reaction conditions, prolonging the preparation process route, increasing the production cycle, etc., and achieves mild reaction conditions and synthetic route Short, avoid strong corrosive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Preparation of S configuration-1-butylpiperidine-2-carboxylic acid
[0044] Add 5g (38.7mmol) of S-configuration 2-piperidinecarboxylic acid and 3.07g (42.6mmol) of n-butyraldehyde to 50ml of methanol, add 234mg (3.9mmol) of glacial acetic acid, stir at room temperature for about 1 hour, then add 1.6g (42.6mmol) sodium borohydride, slowly warming up to 30~40 ℃ and continue stirring reaction at this temperature for 5 hours; Stop reaction, adjust reaction solution pH to 7~8 with 1N sodium hydroxide solution, add 100ml ethyl acetate, The organic layer was collected by extraction and liquid separation, and the organic phase was concentrated by distillation under reduced pressure. The obtained crude product was purified by column chromatography to obtain 4.3 g of S-configuration 1-butylpiperidine-2-carboxylic acid with a yield of 60%. MS:184[M-H]
[0045] 1 H-NMR(400MHz,DMSO-d6),ppm:3.26-3.23(m,1H),3.12-3.08(m,1H),3.0-2.9(m,2H),1.91-1.87(m,1H),1.63 -1.50(m,6H),...
Embodiment 2
[0046] Example 2: Preparation of S configuration-1-butylpiperidine-2-carboxylic acid
[0047]Add 5g (38.7mmol) of S-configuration 2-piperidinecarboxylic acid, 8.37g (116.1mmol) of n-butyraldehyde, and 878mg (7.7mmol) of p-trifluoroacetic acid into 50ml of methanol, stir at room temperature for about 1h, then add 7.3g ( 116.1mmol) sodium cyanoborohydride, continue to stir and react at 30-40°C for 6h; adjust the pH of the reaction solution to 7-8 with 1mol / L sodium hydroxide solution, add 100ml ethyl acetate to extract and separate the liquid, and subtract the organic phase After evaporation and concentration, the resulting crude product was purified by column chromatography to obtain 4.6 g of S-configuration 1-butylpiperidine-2-carboxylic acid with a yield of 65%.
Embodiment 3
[0048] Example 3: Preparation of racemic 1-butylpiperidine-2-carboxylic acid
[0049] Add 5g (38.7mmol) racemic 2-piperidinecarboxylic acid, 4.2g (58.1mmol) n-butyraldehyde, 344mg (2.0mmol) p-toluenesulfonic acid to 50ml ethanol, stir at room temperature for 1h, then add 49.2g (232.2mmol) ) sodium triacetylborohydride, continue to stir and react at 30-40°C for 6h; use 1mol / L sodium hydroxide solution to adjust the pH of the reaction solution to 7-8, add 100ml ethyl acetate to extract and separate the liquid, and evaporate and concentrate the organic phase to obtain The crude product was purified by column chromatography to obtain 4.5 g of racemic 1-butylpiperidine-2-carboxylic acid with a yield of 62.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com