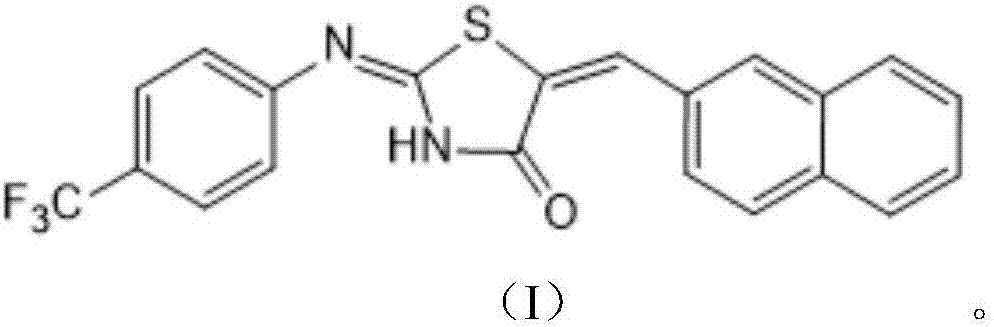
2-(4-trifluoromethyl-phenylimino)-5-(2-naphthyl)methylene-thiazolidin-4-one compound and application thereof
A technology of trifluoromethylaniline and naphthylmethylene, which is applied in the field of medicinal chemistry and achieves the effect of great development and application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of 2-(4-trifluoromethylphenylimino)-5-(2-naphthylmethylene)thiazolidin-4-one
[0023] According to the ratio of HCl:H2O (1:4) dubbed hydrochloric acid solution. Weigh 11.4g (150mmol) of ammonium thiocyanate and dissolve it in 50mL of hydrochloric acid solution, add 12.4mL (100mmol) of 4-trifluoromethylaniline, and heat to 85°C under stirring conditions, the mixture becomes clear. After 12 hours of reaction, TLC Monitor the reaction. After the reaction, the mixture was cooled to room temperature. A viscous oily liquid appeared, extracted with ethyl acetate, and the extract was washed with 10% hydrochloric acid solution, saturated sodium chloride solution, and water in sequence, and the extract was evaporated under reduced pressure. solvent to obtain 4-trifluoromethylphenylthiourea.
[0024] Add 0.906mL (6.0mmol) of 4-trifluoromethylphenylthiourea and 2479mg (30mmol) of anhydrous sodium acetate into 20mL of ethanol. Under stirring conditions, add 1...
Embodiment 2
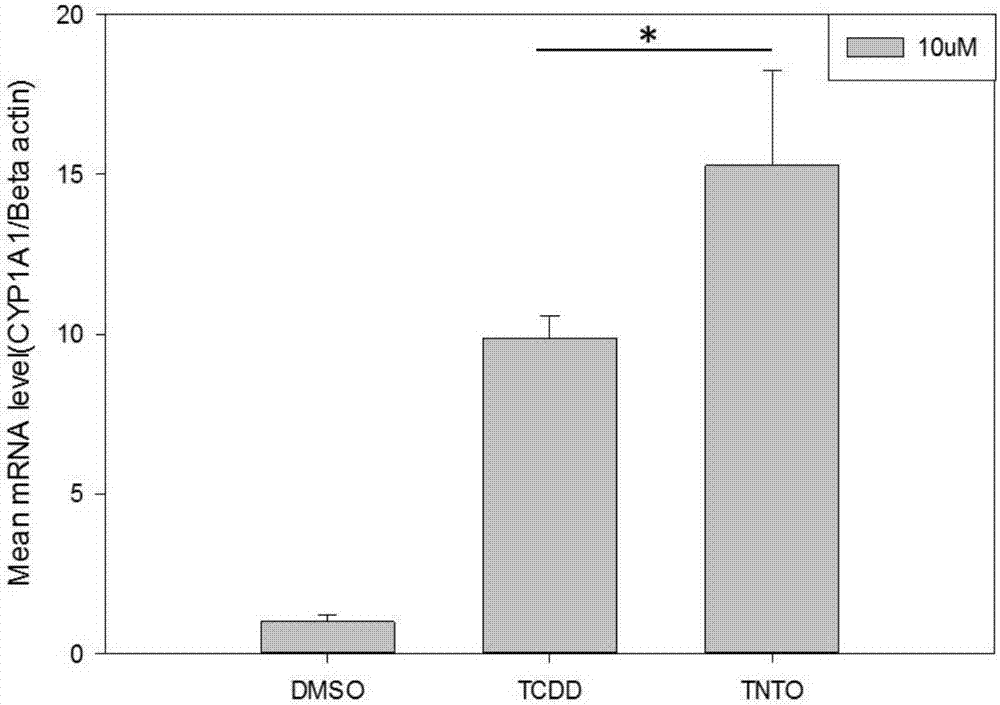
[0030] Example 2: 2-(4-trifluoromethylphenylimino)-5-(2-naphthylmethylene)thiazolidin-4-one compound causes CYP1A1 gene expression up-regulation verification
[0031] 1. Inoculate HepaWT cells into a cell culture dish with a diameter of 60mm at a density of 1×105mL-1. After 24h, the cells were mixed with 2-(4-trifluoromethylanilino)-5-(2-naphthylmethylene)-1,3-thiazol-4-one (10μM), DMSO (0.1% , negative control), TCDD (1nM, positive control) at 37°C with 5% CO2 for 24h.
[0032]2. Extraction of RNA: Collect the cells, discard the medium, and rinse twice with preheated PBS solution. Add an appropriate amount of lysis buffer to lyse the cells, and pipette slowly several times. The lysate was transferred to a spin column placed in a 2 mL collection tube and centrifuged at 13000 rpm for 1 min. Discard the supernatant, and put the spin column into a new 2mL collection tube.
[0033] Add 700 μL of Wash Buffer 1 (absolute ethanol has been added) to the spin column, and centrifuge...
Embodiment 3
[0056] Example 3: Verification that 2-(4-trifluoromethylphenylimino)-5-(2-naphthylmethylene)thiazolidin-4-one compound causes up-regulation of CYP1A1 protein expression
[0057] 1. Dilute HepaWT cells at 1×10 5 mL -1 The density was inoculated into cell culture dishes with a diameter of 60 mm. After 24 h, the cells were mixed with 2-(4-trifluoromethylanilino)-5-(2-naphthylmethylene)-1,3-thiazolidin-4-one (10 μM), DMSO (5 %, negative control), TCDD (1nM, positive control) at 37°C containing 5% CO 2 Under the conditions of the role of 24h.
[0058] Discard the culture medium in the dish, and wash three times with 4°C pre-cooled PBS. After discarding the PBS, place the dish on ice and add 4 mL of PBS. Cells were scraped off with a cell scraper. The remaining cells were observed under a microscope. Suck the PBS into a sterile 15mL centrifuge tube with a sterile hose, and centrifuge at 1000rpm for 5min at 4°C. Centrifuge, remove the supernatant, and obtain a cell pellet. A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com