Preparing method by using unactivated olefin hydrogen trifluoride methylation and application thereof
A technology of methylphenoxy and methoxyphenoxy, which is applied in the field of compound synthesis, can solve the problems of high catalyst consumption, expensive, limited applicability, etc., and achieve wide applicability, high conversion number, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of Compound II-1:
[0036]
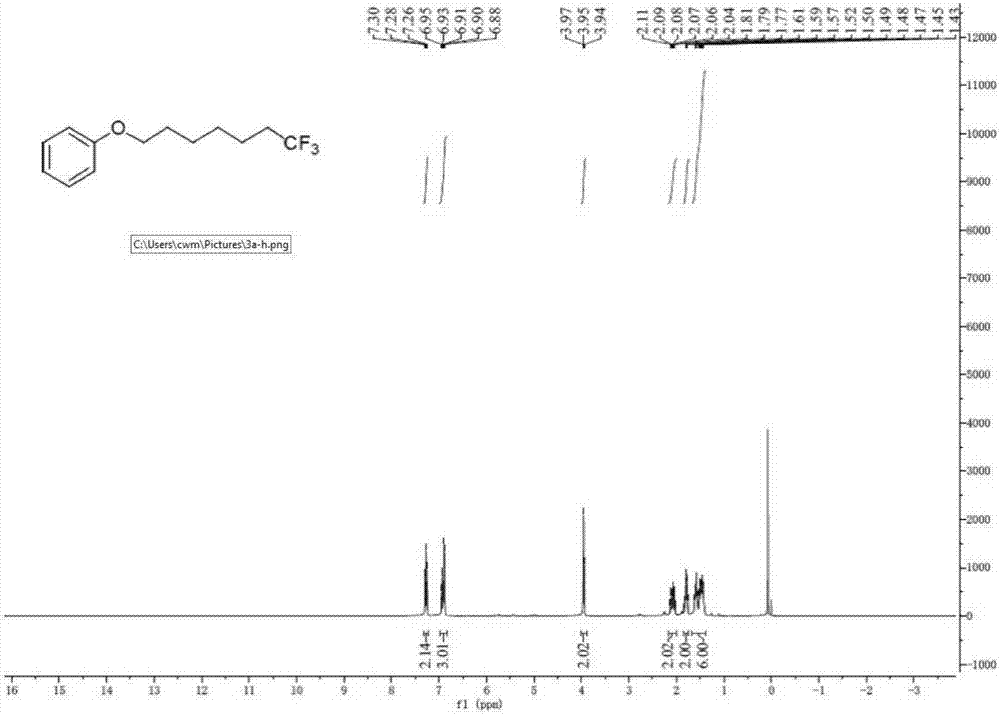
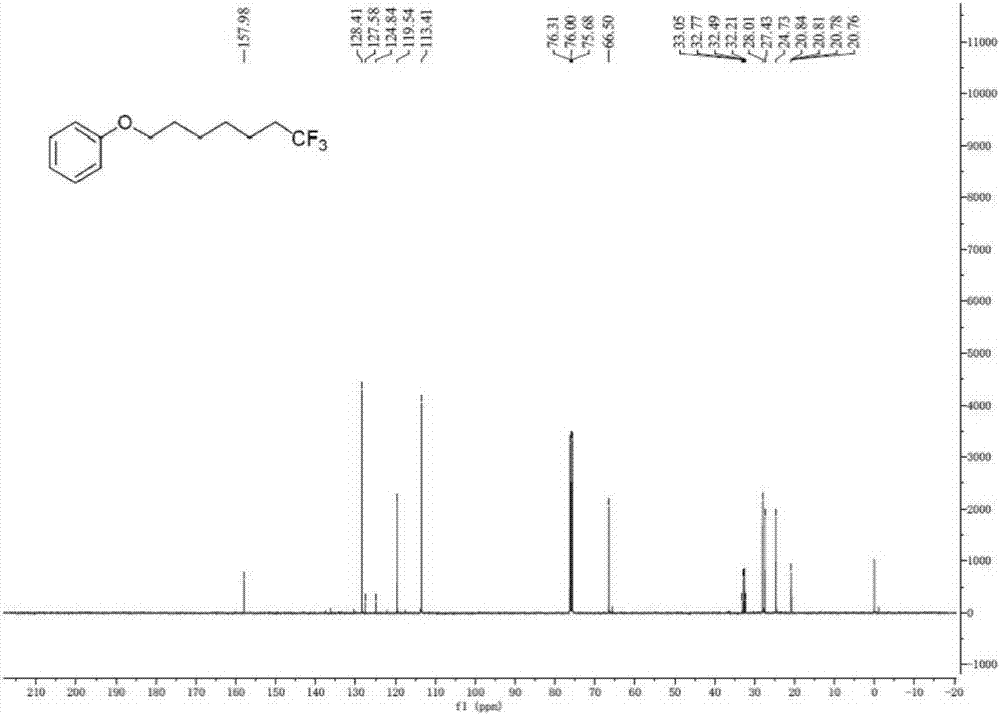
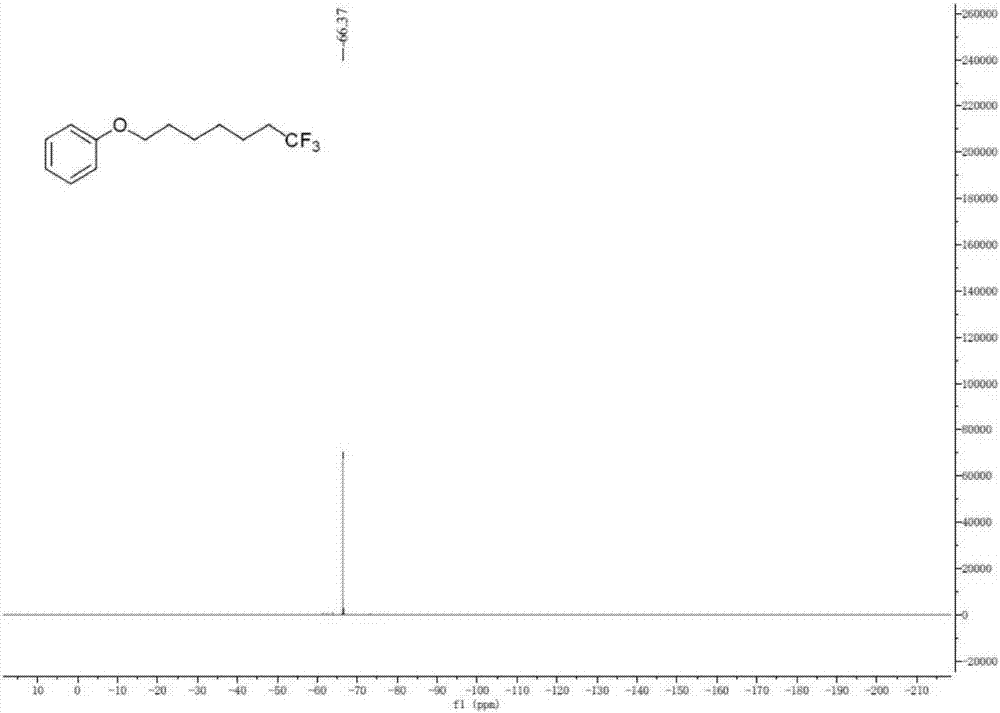
[0037] A. Add unactivated olefin I-1 (R 1 = phenoxy, R 2 =hydrogen, n=4) (44.1mg, 0.25mmol), sodium trifluoromethanesulfinate (78.0mg, 0.50mmol) and photocatalyst Ir[dF(CF 3 )ppy] 2 (dtbpy)PF 6(2mol%, 5.6mg); The ratio of the amount of substance of the sodium trifluoromethanesulfinate and I-1 is 2.0; The photocatalyst Ir[dF(CF 3 )ppy] 2 (dtbpy)PF 6 The amount used is 2.0% of the amount of substance in I-1;
[0038] B, after vacuumizing and changing argon, add solvent (methanol) 3mL; Described solvent is methyl alcohol; The ratio of the milliliter number of described solvent methanol and the amount of substance of I-1 is 12.0;
[0039] C. Irradiate the Schlenk tube with a fluorescent lamp, stir and react; the wavelength range of the fluorescent lamp is visible light, and the power is 36W; the reaction temperature is room temperature (20-25°C, the same below), and the reaction time is 24 hours;
[0040] D. After the reac...
Embodiment 2
[0045] Preparation of compound II-2:
[0046]
[0047] Add unactivated olefin I-2 (R 1 = p-methylphenoxy, R 2 =hydrogen, n=4) (47.6mg, 0.25mmol), sodium trifluoromethylsulfinate (78.0mg, 0.50mmol) and Ir[dF(CF 3 )ppy] 2 (dtbbpy)PF 6 (5.6 mg, 0.005 mmol). After evacuating and changing the argon gas, add 3 mL of solvent (methanol), irradiate the Schlenk tube with a fluorescent lamp, and stir to carry out the reaction. The wavelength range of the fluorescent lamp is visible light, the power is 36W, the reaction temperature is room temperature, and the reaction time is 24 hours. After the reaction, add water to the system to quench the reaction, extract with ethyl acetate (3×10mL), separate the organic phase, and use anhydrous Na 2 SO 4 Dry, filter and remove solvent by rotary evaporation. The residue was subjected to column chromatography with pure petroleum ether, separated and purified to obtain the target product II-253mg, and the yield was 82%.
[0048] 1 H NMR (...
Embodiment 3
[0052] Preparation of Compound II-3:
[0053]
[0054] Add unactivated olefin I-3 (R 1 = p-methoxyphenoxy, R 2 =hydrogen, n=4) (51.6mg, 0.25mmol), sodium trifluoromethylsulfinate (78.0mg, 0.50mmol) and Ir[dF(CF 3 )ppy] 2 (dtbbpy)PF 6 (5.6 mg, 0.005 mmol). After evacuating and changing the argon gas, add 3 mL of solvent (methanol), irradiate the Schlenk tube with a fluorescent lamp, and stir to carry out the reaction. The wavelength range of the fluorescent lamp is visible light, the power is 36W, the reaction temperature is room temperature, and the reaction time is 24 hours. After the reaction, add water to the system to quench the reaction, extract with ethyl acetate (3×10mL), separate the organic phase, and use anhydrous Na 2 SO 4 Dry, filter and remove solvent by rotary evaporation. The residue was subjected to column chromatography with pure petroleum ether, separated and purified to obtain the target product II-356mg, and the yield was 82%.
[0055] 1 H NMR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com