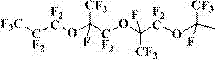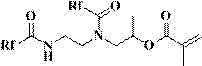Acrylic ester containing perfluoropolyether chain and synthetic method thereof
A technology of perfluoropolyether and acrylate, which is applied in chemical instruments and methods, preparation of sulfonamide, preparation of carboxylic acid amide, etc., can solve problems such as adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The structure of the acrylate containing perfluoropolyether chain synthesized in this embodiment is as follows:
[0061]
[0062] where Rf- is:
[0063]
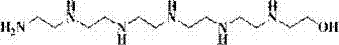
[0064] 1) Synthesis of N-hydroxyethyl perfluoropolyether sulfonamide
[0065] Add 10.4g (0.1mol) of N-hydroxyethylethylenediamine, 50ml of anhydrous ether, and 50ml of methyl acetate into a 500ml reaction bottle, slowly add 142.4g (0.2mol) of all After the addition of methyl fluoropolyether sulfonate was completed, the ice-water bath was removed, and the reaction was continued for 10 hours. Then, the low-boiling components were removed by distillation with a rotary evaporator, and the intermediate N-hydroxyethyl perfluoropolyether sulfonamide was obtained after vacuum drying.
[0066] ) Synthesis of acrylates containing perfluoropolyether chains
[0067] Add 100ml of dichloromethane and 10.8g (0.105mol) of triethylamine to the above-mentioned reaction flask equipped with N-hydroxyethyl perfluoropolyether sulfo...
Embodiment 2
[0075] The structure of the acrylate containing perfluoroether chain synthesized in this embodiment is as follows:
[0076]
[0077] where Rf- is:
[0078]
[0079] 1) Synthesis of N-hydroxyethyl perfluoropolyetheramide
[0080] Add 14.7g (0.1mol) of N-hydroxyethyldiethylenetriamine and 100ml of dichloromethane into a 500ml reaction bottle, and slowly add 153.2g (0.3mol) of perfluoropolyether formic acid methyl in an ice-water bath while stirring After the addition of the ester, the ice-water bath was removed, and the reaction was continued for 10 hours, and then the low-boiling components were removed by distillation with a rotary evaporator, and the intermediate N-hydroxyethyl perfluoropolyetheramide was obtained after vacuum drying. The structure of ethyldiethylenetriamine is as follows:
[0081]
[0082] 2) Synthesis of acrylates containing perfluoropolyether chains
[0083] Add 100ml of anhydrous ether and 10.8g (0.105mol) of triethylamine to the above-mention...
Embodiment 3
[0092] The structure of the acrylate containing perfluoroether chain synthesized in this embodiment is as follows:
[0093]
[0094] where Rf- is:
[0095]
[0096] 1) Synthesis of N-(2-hydroxypropyl) perfluoropolyetheramide
[0097] Add 11.82g (0.1mol) N-(2-hydroxypropyl)-ethylenediamine and 100ml tetrahydrofuran into a 500ml reaction bottle, slowly add 138.03g (0.2mol) perfluoropolyether dropwise under ice-water bath while stirring Ethyl formate, the dropwise addition is completed, remove the ice-water bath, continue the reaction for 10h, then use the rotary evaporator to distill off the low boiling point components, and obtain the intermediate N-(2-hydroxypropyl) perfluoropolyetheramide after vacuum drying, wherein The N-(2-hydroxypropyl)-ethylenediamine structure used is as follows:
[0098]
[0099] 2) Synthesis of acrylates containing perfluoropolyether chains
[0100] Add 100ml of chloroform and 13.57g (0.105mol) of N,N-diisopropylethylamine to the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com