Terpenoid synthase for producing nerolidol and application thereof
A technology of terpenoid synthase and nerolidol, applied in the field of synthetic biology, can solve the problems of high production cost, poisoning by chemical synthesis method, etc., and achieve the effects of reducing research cost, increasing yield, and overcoming low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
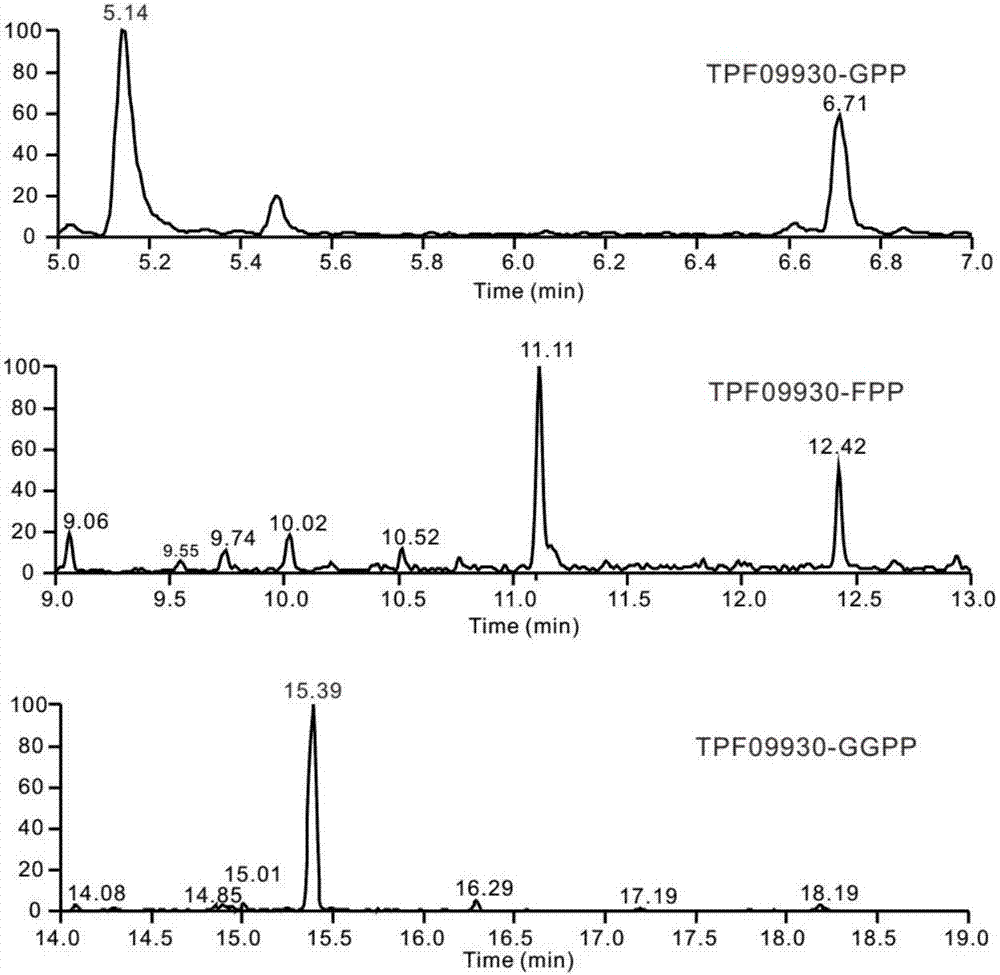
[0028] [Example 1] Verification of the function of terpene synthase in vitro
[0029] 1. Construction of plasmid pGB136
[0030] Using the reverse-transcribed Alternaria alternata TPF6 cDNA as a template, the coding region of TPF09930 was amplified with primers P27 / P29 (see Table 1) and ligated to plasmid pET28a to obtain plasmid pGB136. The nucleotide sequence of TPF09930 is shown in SEQ ID NO:2.
[0031] 2. Protein purification
[0032] The expression vector pGB136 containing the target gene (nucleotide sequence shown in SEQ ID NO: 2) was transformed into the expression host E.coli BL21(DE3). Cultivate overnight at 220 rpm. Transfer to 1L of fresh LB medium containing corresponding antibiotics according to 1% inoculum amount, and cultivate to OD at 37°C and 220rpm 600 About 0.6-0.8, lower the temperature to 16°C, add IPTG with a final concentration of 0.1mM, culture at 16°C, 220rpm for 16-18h. Collect the cells by centrifugation at 8 000 rpm for 5 min, then resuspend th...
Embodiment 2
[0038] [Example 2] Construction of expression vector
[0039] Escherichia coli XL1-blue genomic DNA and Saccharomyces cerevisiae INVSC1 genomic DNA were purified with the Blood and Cell Culture DNA Mini Kit of Qiagen Company.
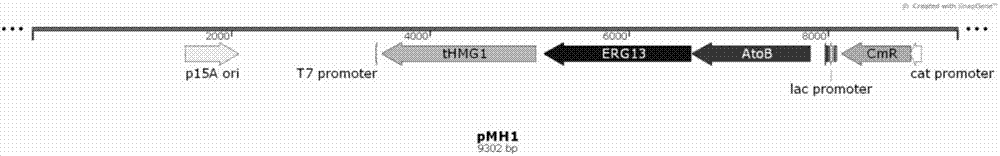
[0040] Plasmid pMH1 contains the first three genes of the mevalonate pathway: atoB gene (acetoacetyl-CoA thioesterase, AM946981.2) from Escherichia coli XL1-blue, erg13 from Saccharomyces cerevisiae INVSC1 (HMG-CoAsynthase, CP005477 .2) and tHMG1 (HMG-CoA reductase, deleted transmembrane region of HMG1, CP005464.2).
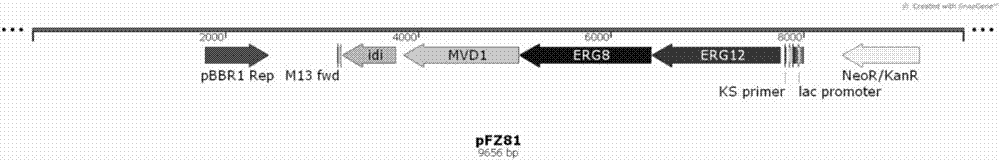
[0041] Plasmid pFZ81 contains the last four genes of the mevalonate pathway: erg12 (mevalonate kinase, CP008027.1), erg8 (mevalonate-5-phosphate kinase, CP005426.1) and mvd1 from Saccharomyces cerevisiae INVSC1 (Mevalonate-5-pyrophosphate kinase, CP005554.2), derived from the idi (isoamyl pyrophosphate isomerase, CP010152.1) gene of Escherichia coli XL1-blue.
[0042] Plasmid pGB286 contains three genes for synthesizing sesquiterpene com...
Embodiment 3
[0057] [Example 3] In vivo synthesis of sesquiterpene compounds derived from TPF09930 in Escherichia coli
[0058] In order to produce sesquiterpene compounds, two plasmids of the mevalonate pathway, pMH1 and pFZ81, were simultaneously transformed into E. coli BL21(DE3) to obtain BL21(DE3) / pMH1 / pFZ81, named PS, and then pGB286 was transformed into the strain In PS, strain N1 was obtained, and then single clones were picked up into 10 mL of LB medium (containing 100 μg / mL ampicillin, 50 μg / mL kanamycin and 34 μg / mL chloramphenicol at the same time), 37 ° C, 220 rpm overnight Cultivated, then inoculated into the same fresh medium at 1% inoculum at 37°C, continued to culture at 220rpm until the OD600 was about 0.6 to 0.8, cooled to 16°C and added IPTG with a final concentration of 0.1mM to induce expression. After 18 hours, the temperature was raised to 28 °C for fermentation for 72 hours, and then fermentation and product extraction were carried out. The bacterial cells and ferm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com