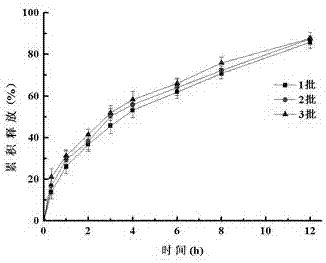
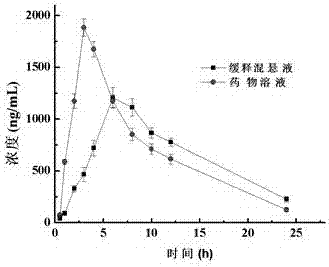
Preparation method of carbinoxamine maleate sustained-release suspension
A slow-release suspension and slow-release technology, which is applied in the fields of pharmaceutical formulations, digestive system, respiratory system diseases, etc., can solve problems such as low production capacity and technical difficulties, achieve convenient operation, prolong the preparation cycle, and stabilize blood drug concentration Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the preparation process of carbinoxamine maleate sustained-release suspension
[0047] (1) Preparation of drug resin:
[0048] Dissolve 10 g of carbinoxamine maleate in 1 L of deionized water, stir on a magnetic stirrer at 1000 rpm, add 20 g of Amberlite ® IRP69, regular sampling, determination of the concentration of the drug in the solution. When the drug concentration no longer decreases with time, the equilibrium is reached, and the unbound drug on the surface of the resin is washed away with deionized water, and dried at 60° C. for 2 hours to obtain the carbinoxamine maleate drug-loaded resin.
[0049] (2) Impregnation of drug resin:
[0050] Take 30g of carbinoxamine maleate drug-loaded resin, add it to 1L of 10% PEG4000 aqueous solution, stir for 1 hour, dry at 60° C. for 0.5 hour, and pass through a 100-mesh sieve to obtain impregnated carbinoxamine maleate drug resin.
[0051] (3) Preparation of drug resin microcapsules:
[0052] ① Preparation ...
Embodiment 2
[0066] Embodiment 2: the preparation process of carbinoxamine maleate sustained-release suspension
[0067] (1) Preparation of drug resin:
[0068] Dissolve 10 g of carbinoxamine maleate in 1 L of deionized water, stir on a magnetic stirrer at 1000 rpm, add 20 g of Amberlite ® IRP88, regular sampling, determination of the concentration of the drug in the solution. Equilibrium was reached when the drug concentration no longer decreased with time, and the unbound drug on the surface of the resin was washed away with deionized water, and dried at 40° C. for 2 hours to obtain the carbinoxamine maleate drug-loaded resin.
[0069] (2) Impregnation of drug resin
[0070] Get 30g carbinoxamine maleate drug-loaded resin, join in the aqueous solution of 1L 30% glycerin, stir 1 hour, 60 ℃ dry 0.5 hour, pass through 100 mesh sieves to obtain the carbinoxamine maleate drug resin impregnated.
[0071] (4) Preparation of drug resin microcapsules
[0072] ① Preparation of dispersed phase...
Embodiment 3
[0086] Embodiment 3: the preparation process of carbinoxamine maleate sustained-release suspension
[0087] (1) Preparation of drug resin
[0088] Dissolve 10 g of carbinoxamine maleate in 1 L of deionized water, stir on a magnetic stirrer at 1000 rpm, add 20 g of Amberlite ® IRP69, regular sampling, determination of the concentration of the drug in the solution. When the drug concentration no longer decreases with time, the equilibrium is reached, and the unbound drug on the surface of the resin is washed away with deionized water, and dried at 50° C. for 2 hours to obtain the carbinoxamine maleate drug-loaded resin.
[0089] (2) Impregnation of drug resin
[0090] Take 30g of carbinoxamine maleate drug-loaded resin, add it to 1L of 20% PEG4000 aqueous solution, stir for 1 hour, dry at 60° C. for 0.5 hour, and pass through a 100-mesh sieve to obtain impregnated carbinoxamine maleate drug resin.
[0091] (5) Preparation of drug resin microcapsules
[0092] ① Preparation o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com