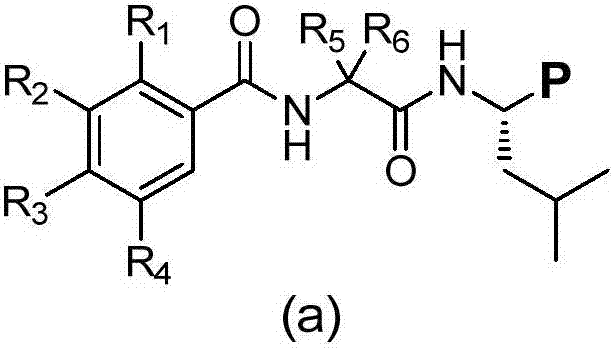
Deuterated dipeptide boric acids or esters thereof, and synthetic methods and uses of the compounds
A technology of dipeptide boronic acid and boric acid esters, which is applied in the field of deuterated dipeptide boronic acid or its ester compounds and their synthesis, and can solve neuropathy and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0155] Example 1: (S)-N-(2,5-dichlorobenzoyl)-2,2-dideuterateacetamide-D-leucine boronic acid citrate (compound NNU-459). synthetic route:
[0156]
[0157] Preparation of (S)-N-(2,5-dichlorobenzoyl)glycine (Ⅳ):
[0158] 2,5-Dichlorobenzoic acid (7.6 g, 40 mmol) and HOBt (8.1 g, 40 mmol) were dissolved in CH 2 Cl 2 (200mL), react at -10°C for 10min, add EDC.HCl (11.5g, 60mmol) and react for 30min, add compound II (5g, 40mmol), add DIPEA (18.1g, 140mmol) after reacting for 10min, and react for 20min Rise to room temperature to react overnight. TLC detects reaction, respectively with 10% hydrochloric acid solution (200mL), 5% NaHCO 3 the solution
[0159]
[0160] (200mL) and saturated brine (2×200mL) washing, CH 2 Cl 2 Anhydrous Na 2 SO 4 After drying, filtering, and evaporating the solvent under reduced pressure, 9.32 g of an oily compound was obtained, with a yield of 88.9%.
[0161] Compound III (129 mg, 0.31 mmol) obtained in the previous step was dissolved ...
Embodiment 2
[0171] Example 2: ((R)-1-(2-(2,5-benzamido)-2,2-didedeuteroacetamido)-3-methylbutyl)boronic acid diethanolamine ester (compound NNU- 458)
[0172]
[0173] According to the method described in Example 1, the difference is that diethanolamine was used instead of citric acid to prepare the target compound. 1 H NMR (400MHz, DMSO) 0.85-0.74 (-CH 3 ,m,6H),1.33-1.16(-CH 2 ,m,2H),1.64-1.51(-CH,m,1H),2.98-2.63(-CH 2 ,m,4H),3.13(-CH,td,J 1 =14.0Hz,J 2 =7.1Hz,1H),3.67(-CH 2 ,dd,J 1 =11.4Hz,J 2 =5.8Hz,4H),5.24(-NH,t,J=22.6Hz,1H),6.58(-NH,d,J=28.1Hz,1H),7.01(-NH,d,J=8.4Hz,1H ),7.65-7.43(-Ph,m,3H),8.79(d,J=36.3Hz,1H).MS(ESI):observed:m / z 432.3[M+H] +
Embodiment 3
[0174] Embodiment 3: pharmacokinetic evaluation in rats
[0175] Twelve SD male rats, weighing 220±20g, were randomly divided into four groups.
[0176] The two groups according to 0.500mg·kg -1 Dose: 0.100mg·mL by tail vein injection -1 For NNU-459 and MLN-9708, about 0.200 mL of blood was collected from the jugular vein at 10 min, 20 min, 30 min, 1 h, 2 h, 4 h, 8 h, 12 h, 24 h and 36 h before administration and after administration, and placed in a -K 2 In a test tube, the plasma was separated after high-speed centrifugation (7800×g) for 5 minutes, and stored at -15°C to -35°C. It is used to compare the pharmacokinetic difference between NNU-459 and MLN-9708 administered by intravenous injection.
[0177] The other two groups received 1.50mg·kg -1 The dose of 0.150mg·mL was given by intragastric administration -1 For NNU-459 and MLN-9708, about 0.200 mL of blood was collected from the jugular vein at 5 min, 10 min, 20 min, 30 min, 1 h, 2 h, 4 h, 8 h, 12 h, 24 h and 36 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com