Primer combination for detecting infectious diarrhea pathogen and kit thereof
A primer composition and a technology for infectious diarrhea, applied in the field of nucleic acid amplification, can solve the problems of high false negative rate and false positive rate, difficulty in detecting difficult-to-cultivate pathogens, and long detection time (generally about 2-3 days).
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] This example describes the microfluidic chip used in the present invention.
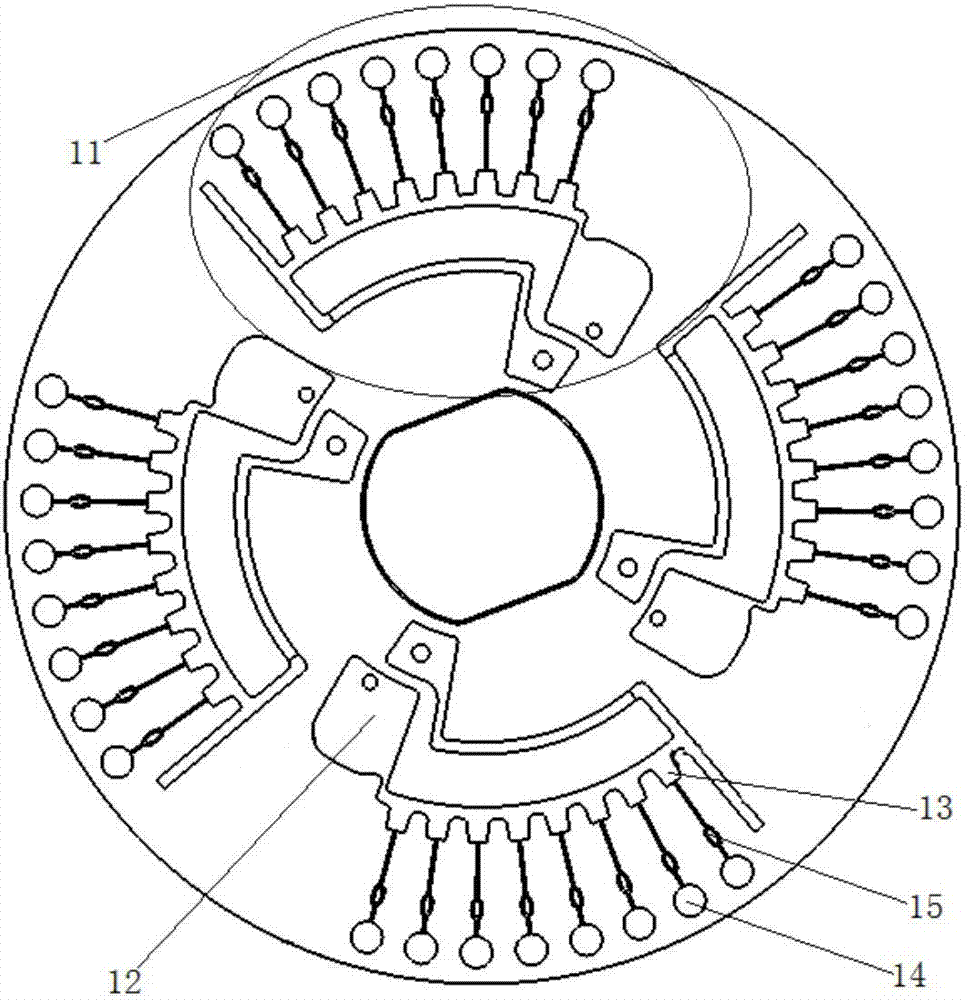
[0057] Such as figure 1 As shown, the microfluidic chip used in the present invention is a disc-shaped microfluidic chip, which includes 4 reaction detection areas 11, and each reaction detection area 11 includes a sequentially connected sample pool 12, distribution pool 13, capillary micro The valve 15 and the amplification pool 16, the sampling pool 12 communicates with the distribution pool 13 through an arc-shaped channel, the sampling pool is provided with a sampling hole, and the distribution pool 13 communicates with the waste liquid pool and the exhaust hole through an arc-shaped channel ; Each reaction detection area 11 is equipped with 8 amplification pools 16 .
[0058] Among them, the main function of the sampling pool 12 is to load the reaction solution; the main function of the distribution pool 13 is to evenly distribute the reaction solution to the amplification pool; the ampl...
Embodiment 2
[0061] This example is the primer composition and kit used in the present invention for detecting infectious diarrhea pathogens.
[0062] The primer composition of the present invention includes at least one of six primer sets packaged independently. The infectious diarrhea pathogen includes at least one of the following six pathogens: rotavirus, enteric adenovirus, norovirus, salmonella, shigella, and campylobacter jejuni. The six primer sets corresponding to the above-mentioned pathogens are rotavirus primer set, intestinal adenovirus primer set, norovirus primer set, salmonella primer set, shigella primer set and campylobacter jejuni primer set. Included is an internal control primer set for amplifying the human hemoglobin-beta chain. See Table 1 for details of the primer sequences of the above primer sets.
[0063] The present invention relates to a kit containing the above primer composition, which also includes the microfluidic chip coated with the above primer set as ...
Embodiment 3
[0066] This embodiment is a method for detecting infectious diarrhea pathogens using the primer composition and kit described in Embodiment 2, which includes the following steps:
[0067] 1. Coating of primer composition;
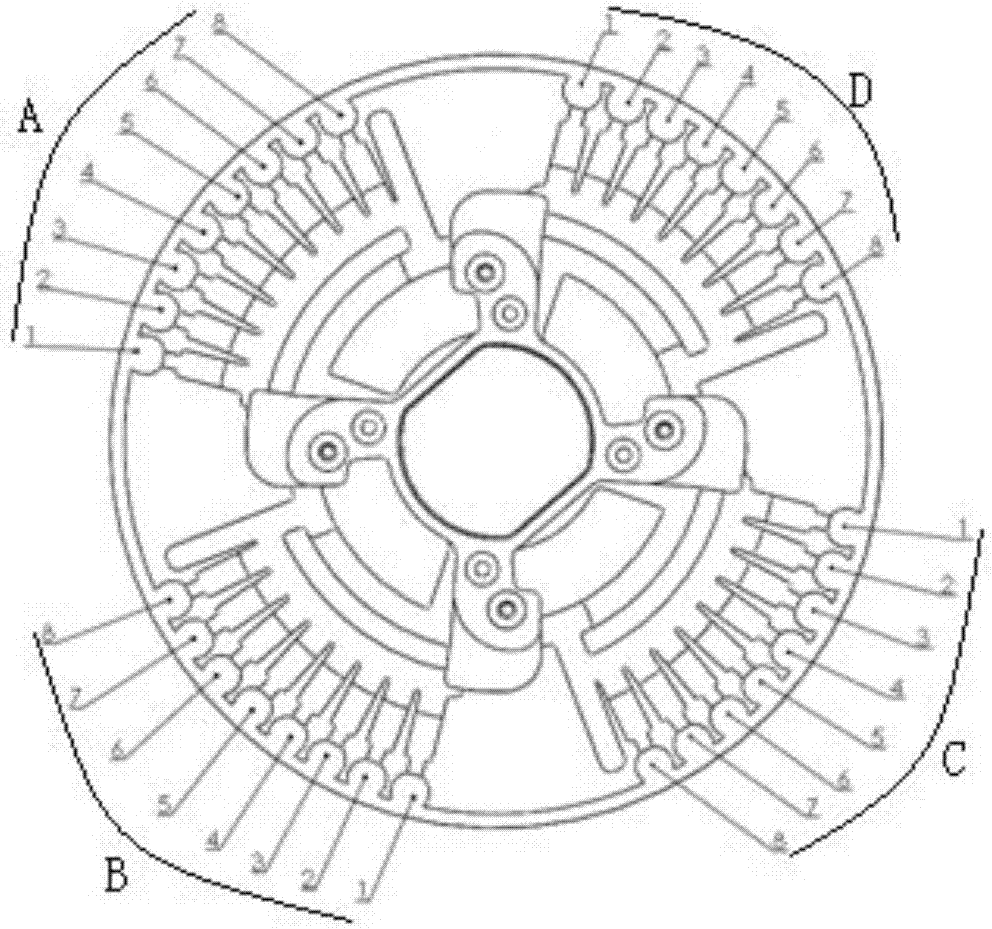
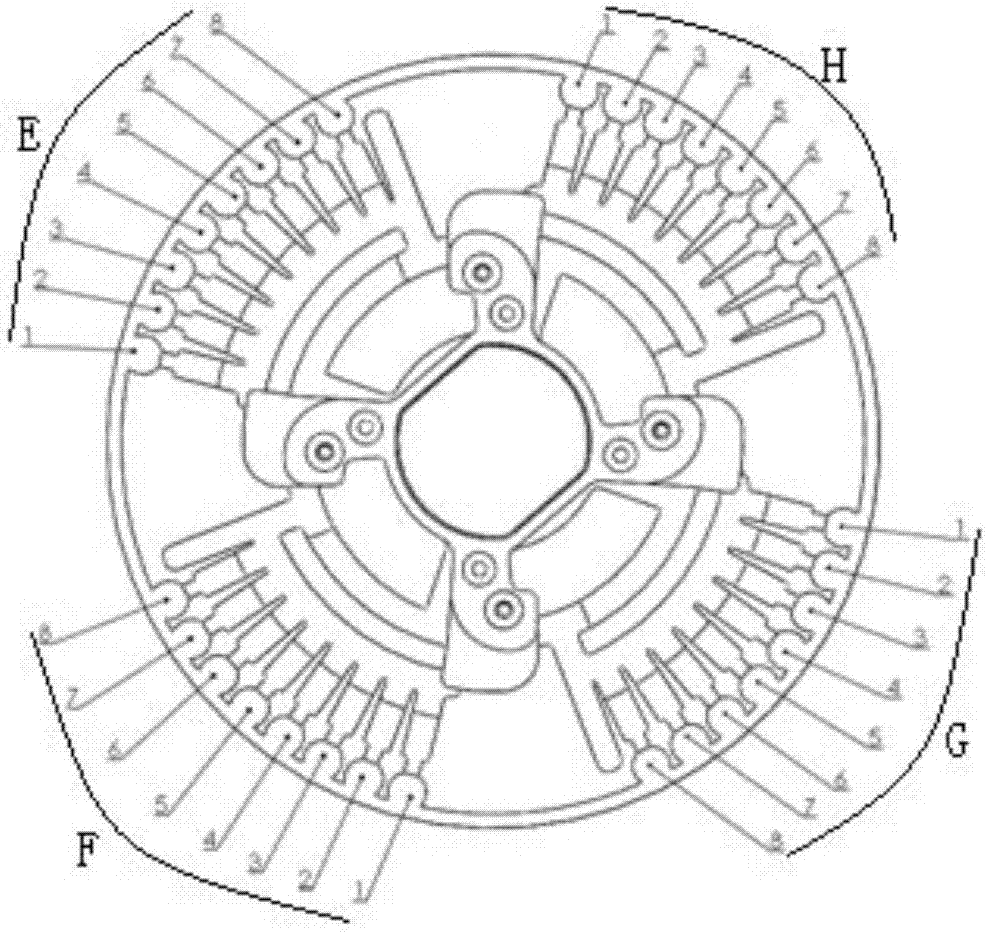
[0068] Such as figure 2 and image 3 As indicated, the rotavirus primer set, intestinal adenovirus primer set, norovirus primer set, salmonella primer set, shigella primer set, campylobacter jejuni primer set, and internal control primer set were mixed with sucrose respectively to prepare into a corresponding mixed solution, and the final concentrations of each primer group and sucrose in the mixed solution were 0.15 μM and 1.0% (mass percentage); get 1 μ L of the mixed solution and put it into the corresponding amplification pool of the microfluidic chip (amplification pool 1, 2, 3, 4, 5, 6, 8), the amplification pool 7 stores RNase-free water, and the microfluidic chip is dried in a 37°C oven, pressed and sealed, and after stamping and vacuuming, the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com