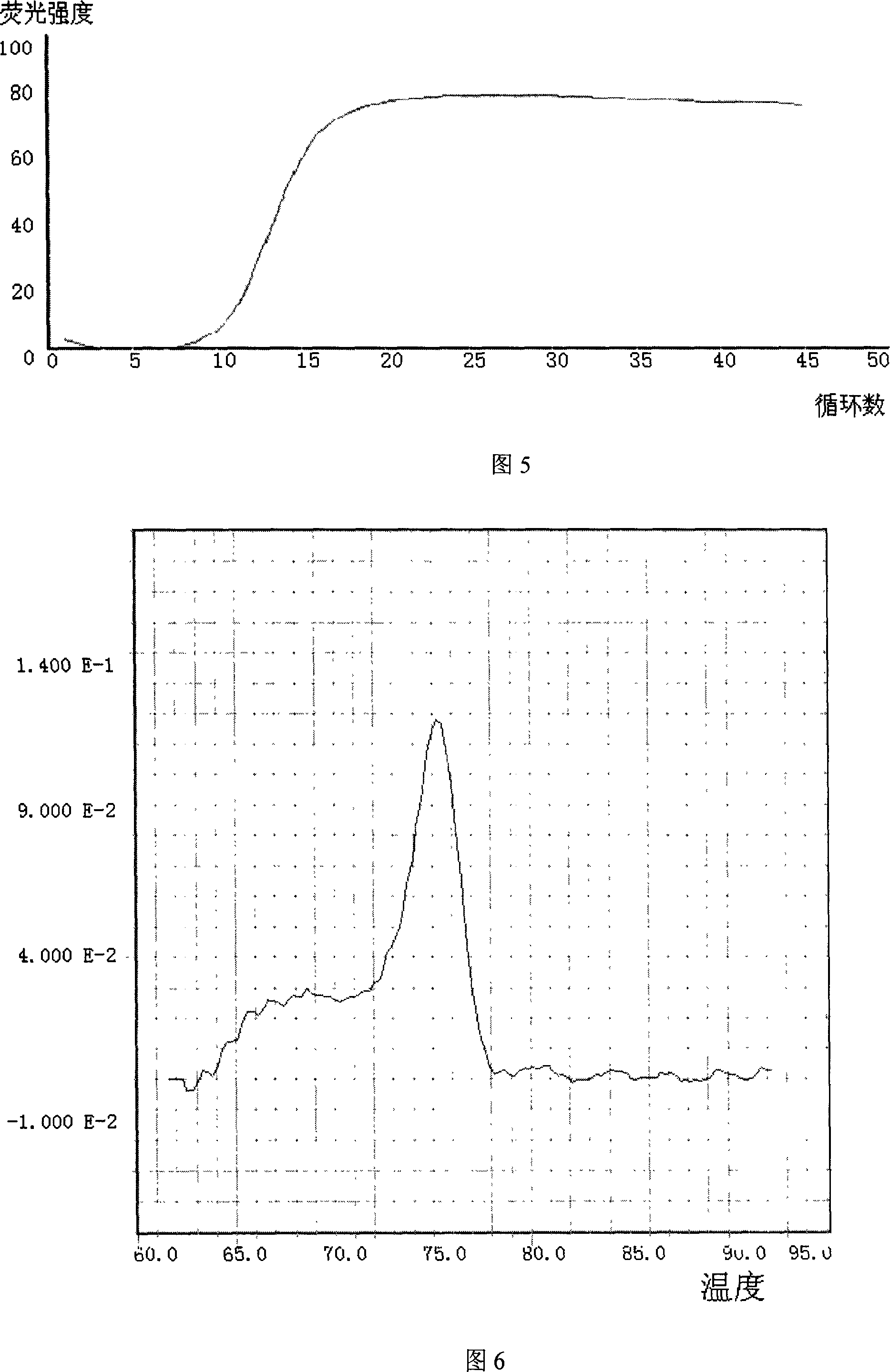
Patents
Literature
171 results about "Shigella sonnei Dysentery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The causative agent of human shigellosis, Shigella causes disease in primates, but not in other mammals. It is only naturally found in humans and gorillas. During infection, it typically causes dysentery. Shigella is one of the leading bacterial causes of diarrhea worldwide, causing an estimated 80–165 million cases.
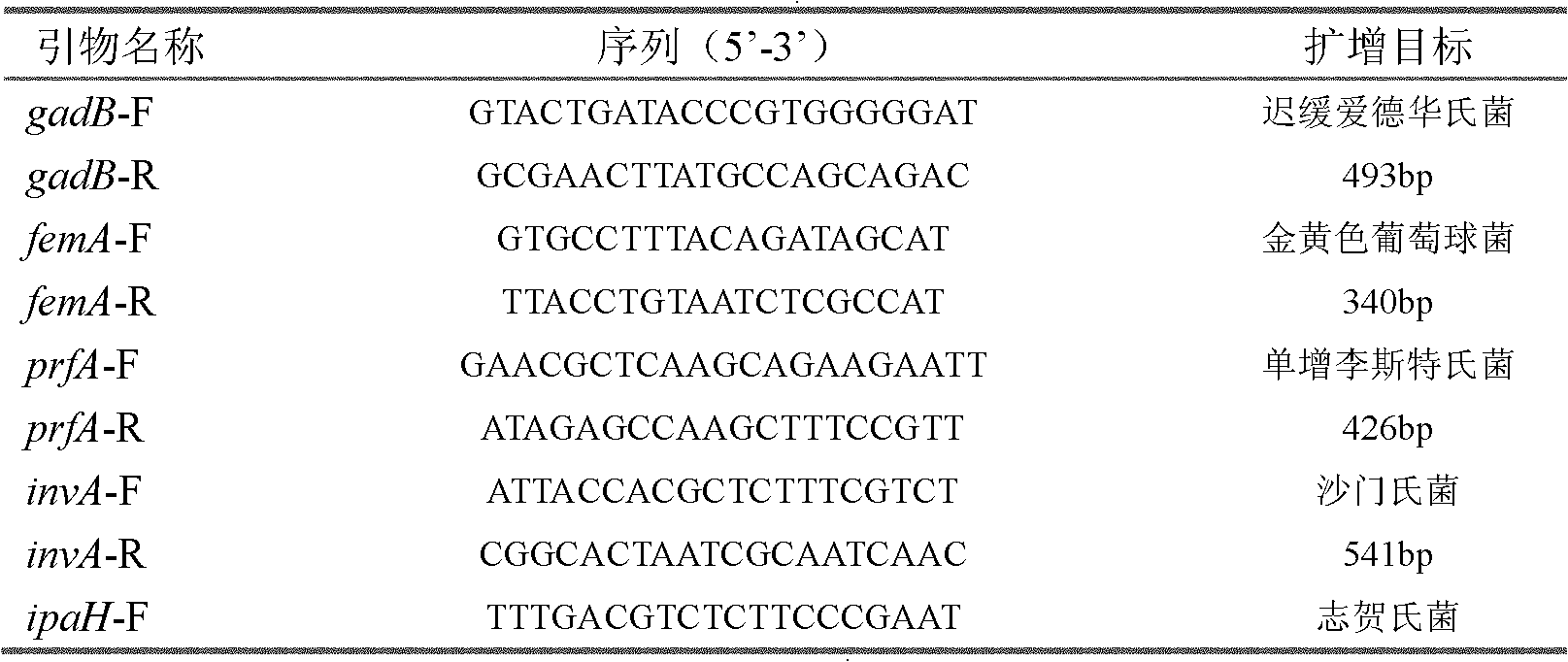
Primer, detection method and detection reagent kit for detecting shigella
InactiveCN101153326AStrong specificityIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationFood borneLoop-mediated isothermal amplification
The invention relates to a technique for fast detecting food-borne pathogens based on a loop-mediated isothermal amplification, LAMP technique. A primer for detection of shigella can augment the specific base sequence of a target gene which is the ipaH-GenBank (accession no. M32063) of the shigella, and the primer is complementary to a part of or a complementary chain of the nucleic acid sequence on the 1095-1299bp loci on the target gene. The invention provides a primer unit having specificity to a specific gene fragment of the shigella, and through detecting whether or not the detecting specimen in a reagent box of the primer unit contains the specific gene fragment of the shigella, determines whether the shigella exists in the specimen or not.
Owner:ZHUHAI DISEASE PREVENTION & CONTROL CENT
Protein-protein interactions between Shigella flexneri polypeptides and mammalian polypeptides
InactiveUS20030055220A1Treat and prevent bacillary dysenteryMore effective and better targeted therapeutic applicationsPeptide/protein ingredientsMicrobiological testing/measurementNucleotideADAMTS Proteins
The present invention relates to protein-protein interactions between Shigella polypeptides and mammalian polypeptides. More specifically, the present invention relates to complexes of polypeptides or polynucleotides encoding the polypeptides, fragments of the polypeptides, antibodies to the complexes, Selected Interacting Domains (SID(R)) which are identified due to the protein-protein interactions, methods for screening drugs for agents which modulate the interaction of proteins and pharmaceutical compositions that are capable of modulating the protein-protein interactions.
Owner:HYBRIGENICS SA
Pathogenic microorganism DNA detecting chip and preparation method and application thereof
InactiveCN101113476ASensitive hybridization detectionAccurate readingMicrobiological testing/measurementAgainst vector-borne diseasesESCHERICHIA COLI ANTIGENNucleic Acid Probes
The invention relates to a pathogenic microorganism DNA detection chip and the chip comprises a carrier and a nucleic acid probe on the carrier. The nucleic acid probe is provided with a target detection probe used for detecting target gene of pathogenic microorganism. The pathogenic microorganism comprises but not limits to vibrio cholerae, pathogenic escherichia coli, campylobacter jejuni, Yersinia enterocolitica, parahemolytic vibrio, salmonella, and shigella and Listeria monocytogenes. The invention also relates to a preparation method of the pathogenic microorganism DNA detection chip and provides applications of the pathogenic microorganism DNA detection chip in detection of pathogenic microorganism. Also a pathogenic microorganism DNA detection chip kit is provided.
Owner:ICDC CHINA CDC
Gene chip and kit for detecting common pathogen in dairy products
ActiveCN101240335AImprove accuracyGood repeatabilityBioreactor/fermenter combinationsBiological substance pretreatmentsKlebsiella oxytocaStaphylococcus aureus
The present invention provides a gene chip for detecting common pathogen in dairy, which comprises a solid-phase vector and an oligonucleotide probe fixed on the solid-phase vector, wherein the oligonucleotide probe includes DNA fragment or its complementary DNA or RNA sequence selected from E. sakazakii, streptococcus pyogenes, staphylococcus aureus, Klebsiella pneumoniae, Klebsiella oxytoca, Listeria monocytogenes, salmonella and 16S-23S rDNA intergenic spacer region of Bacillus cereus, as well as ipaH gene of Shigella or gyrB gene of citrobacter freundii. The invention further provides a reagent box which uses the gene chip to detect pathogen in dairy. The gene chip and the reagent box of the invention for detecting pathogen in dairy are easy to operate, highly precise and greatly repeatable.
Owner:TIANJIN BIOCHIP TECH CO LTD +1
Multiplex LAMP detection primer, kit and method for six food-borne pathogenic bacteria in fruits and vegetables
InactiveCN107022644AStrong specificityHigh amplification efficiencyMicrobiological testing/measurementMicroorganism based processesEscherichia coliBacteroides
The invention discloses a multiplex LAMP detection primer, kit and method for six food-borne pathogenic bacteria in fruits and vegetables and belongs to the technical field of bacterial gene detection. A rapid detection primer set for the six pathogenic bacteria including listeria monocytogenes, enterobacter sakazakii, shigella spp, staphylococcus aureus, salmonella spp and escherichia coli O157:H7 is designed, and multiplex LAMP reaction is performed on the genome DNA of the bacteria extracted from a sample to be detected in the same reaction system by use of the detection kit including the primer set to determine whether the sample contains the six food-borne pathogenic bacteria or not. The multiplex LAMP detection primer is high in specificity and sensitivity and can accurately detect the genome DNA of the six food-borne pathogenic bacteria in the same reaction system, can realize simple and convenient, quick and accurate detection, is suitable for on-site rapid detection and has significance on improving the pathogenic bacterium analysis and detection technology and the fruit and vegetable edible quality security.
Owner:INST OF QUALITY STANDARDS & TESTING TECH FOR AGRO PROD OF SHANDONG ACADEMY OF AGRI SCI
Culture medium capable of simultaneously enriching five kinds of food-borne pathogenic bacteria and preparation method for culture medium
ActiveCN102660474AImprove detection efficiencyLow costBacteriaMicrobiological testing/measurementEscherichia coliFood borne
The invention relates to a culture medium for simultaneous composite enrichment of five kinds of food-borne pathogenic bacteria, namely Salmonella, Escherichia coli, Staphylococcus aureus, Listeria monocytogenes and Shigella, and a preparation method for the culture medium. Food-borne pathogenic bacteria are a significant reason to cause food positioning, so the rapid and accurate detection of the food-borne pathogenic bacteria has an important practical significance for preventing and controlling food safety incidents. The culture medium is characterized by comprising the following components: 10.0 g of peptone, 10.0 g of sodium chloride, 9.0 g of disodium hydrogen phosphate, 1.5 g of monopotassium phosphate, 0.1 g of cholate, 0.1 mg of potassium tellurite, 1.0 g of lithium chloride, 3.0 g of glucose, 2.0 g of mannitol, 2.5 g of sodium pyruvate, 1.0 g of aesculin and 1,000 mL of distilled water, wherein the pH value is 7.1 to 7.5. The culture medium can simultaneously enrich five kinds of target pathogenic bacteria, can be used for separation and identification of target bacteria, can also be used for the molecular detection of multiple pathogenic bacteria on the same detection platform, provides technical support for a method for rapidly detecting five kinds of pathogenic bacteria in food, and meets the requirement of simultaneous detection of five kinds of food-borne pathogenic bacteria.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES +1
Gene chip of main pathogenic microorganism in drinking water and testing kit
InactiveCN101748192ALow costImprove throughputMicrobiological testing/measurementAgainst vector-borne diseasesEscherichia coliBacteroides
The invention provides a gene chip of main pathogenic microorganism in drinking water and a testing kit, which mainly aims at 11 kinds of bacteria of colibacillus / Shigella, salmonella, vibrio cholera, vibrio parahaemolyticus, staphylococcus aureus, enterococcus faecails, pseudomonas aeruginosa, legionella pneumophilia, pneumobacillus, yersinia enterocolitica and the like, and L.interrogans. The gene chip comprises a solid phase carrier and a oligonucleotide probe fixed on the solid phase carrier, wherein the oligonucleotide probe contains gyrB gene with tremendous evolutionary advantage, ITS gene and DNA segment selected from 16srRNA gene or complementary DNA segment. The gene chip and the testing kit of the invention can test the main pathogenic microorganism in drinking water, and has the characteristics of simple operation, high throughput, high accuracy, strong repeatability and the like, and can be used for clinical test for the water quality monitoring department.
Owner:NANKAI UNIV
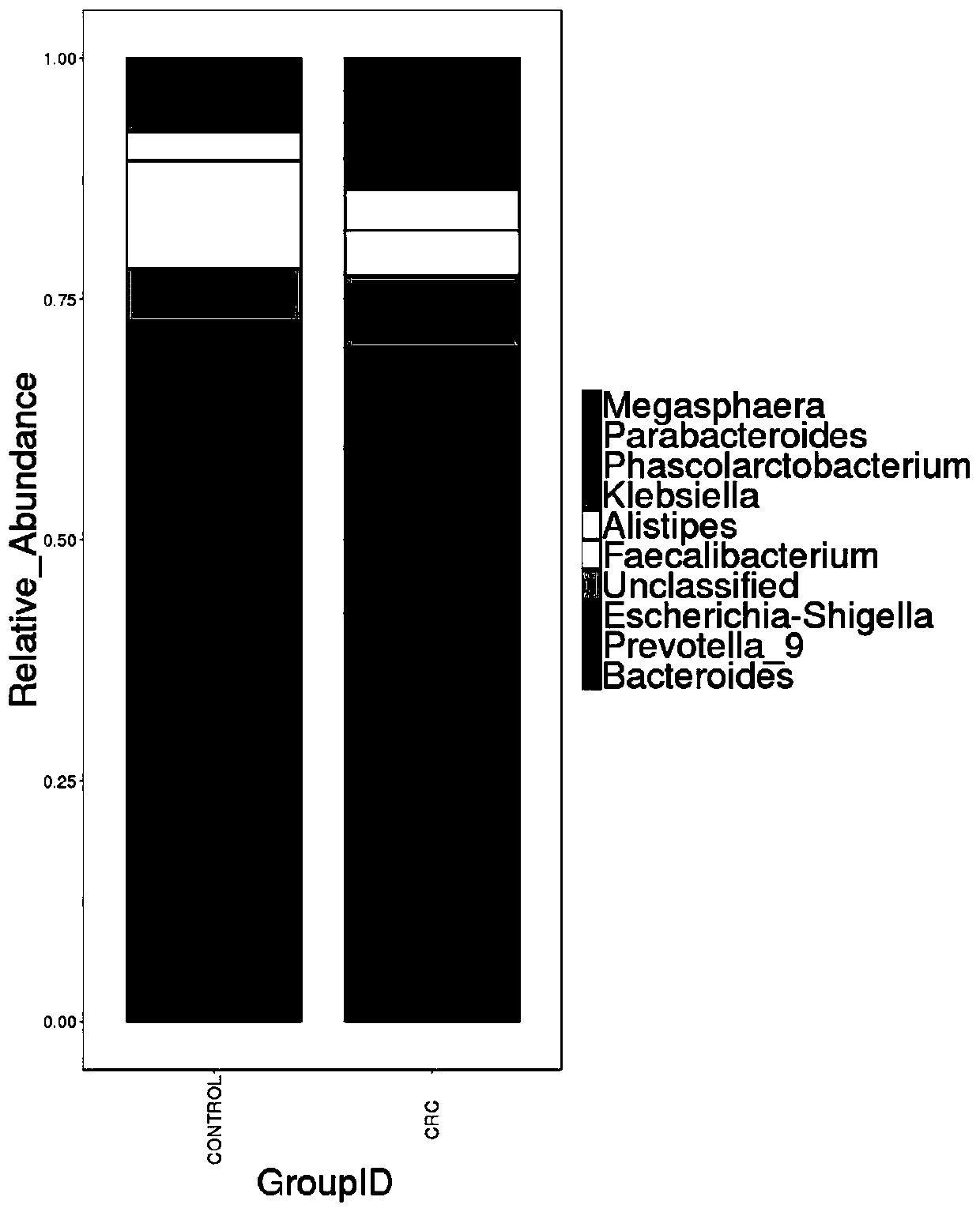
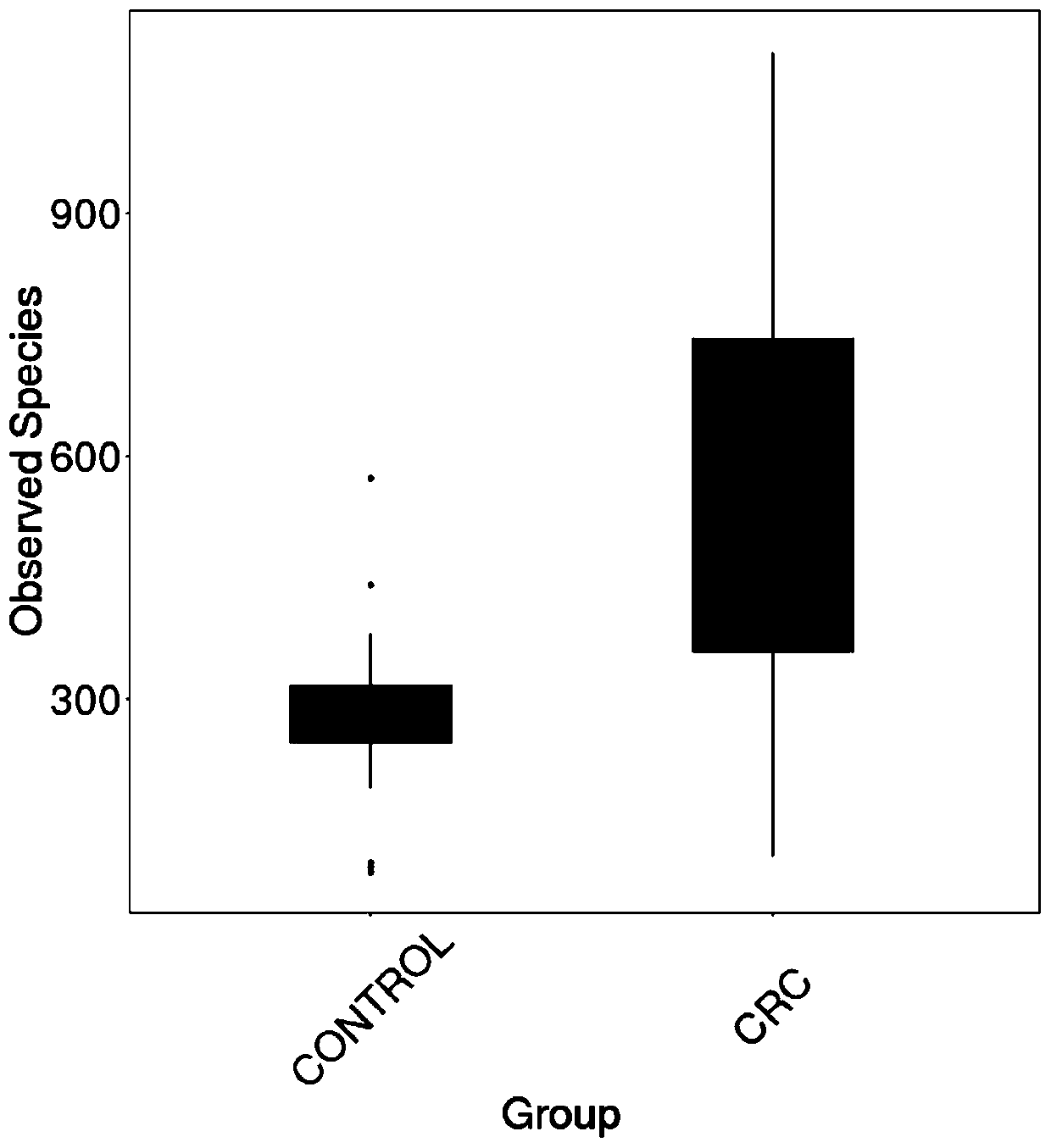
Intestinal flora maker for intestinal cancer and application of intestinal flora maker
InactiveCN110408699AGood forecastMicrobiological testing/measurementGut floraCorynebacterium amycolatum
The invention discloses an intestinal flora maker for intestinal cancer and an application of the intestinal flora maker, and belongs to the technical fields of microorganisms and genetic engineering.The intestinal flora maker comprises megasphaera, veillenella, streptococci, bilophila sp., haemophilus, corynebacterium or Escherichia-Shigella. Through detection by the intestinal flora maker, risks of individuals suffering from intestinal cancer can be assessed, and auxiliary reference can be provided for disease diagnosis and prognosis monitoring.
Owner:FUJIAN HEALTH COLLEGE
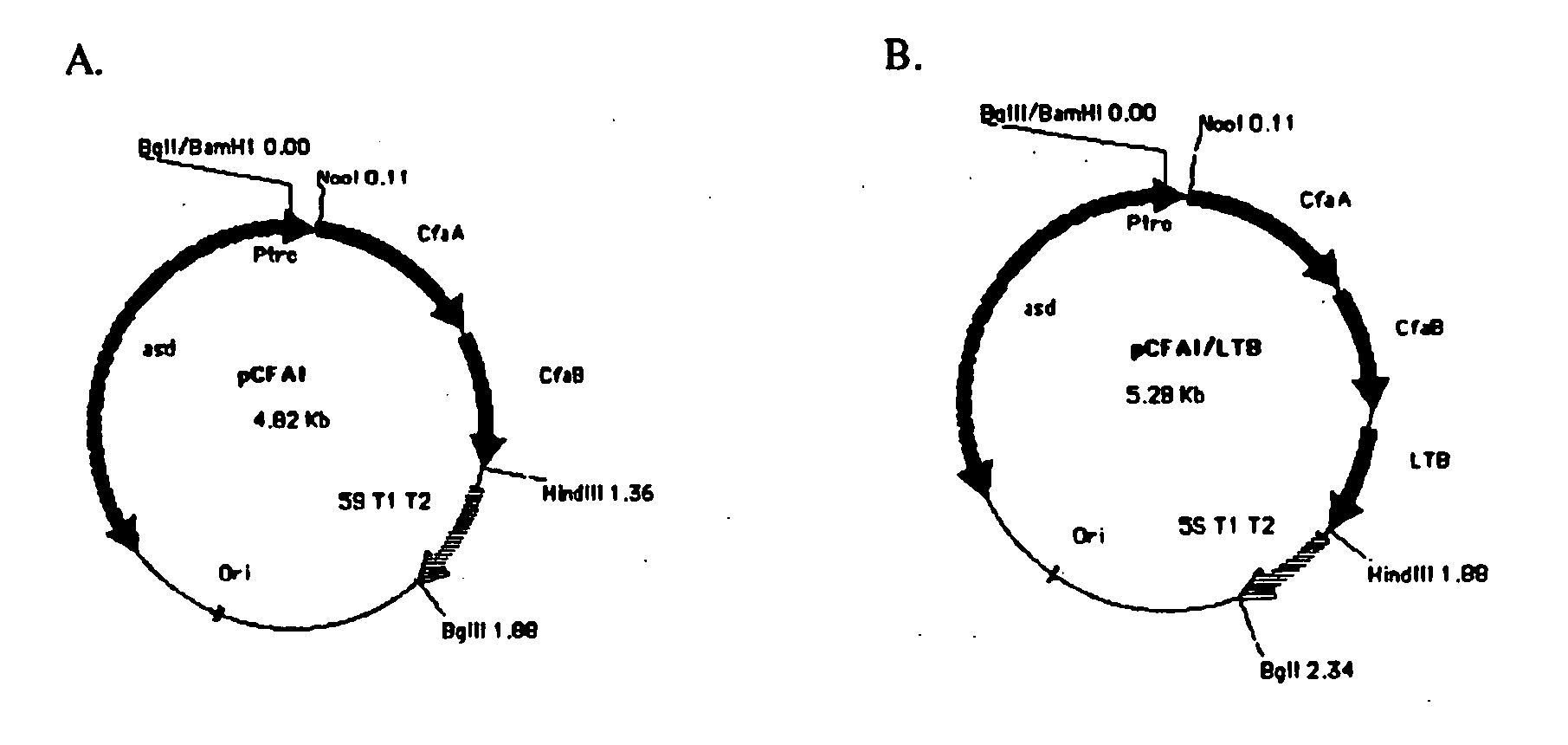
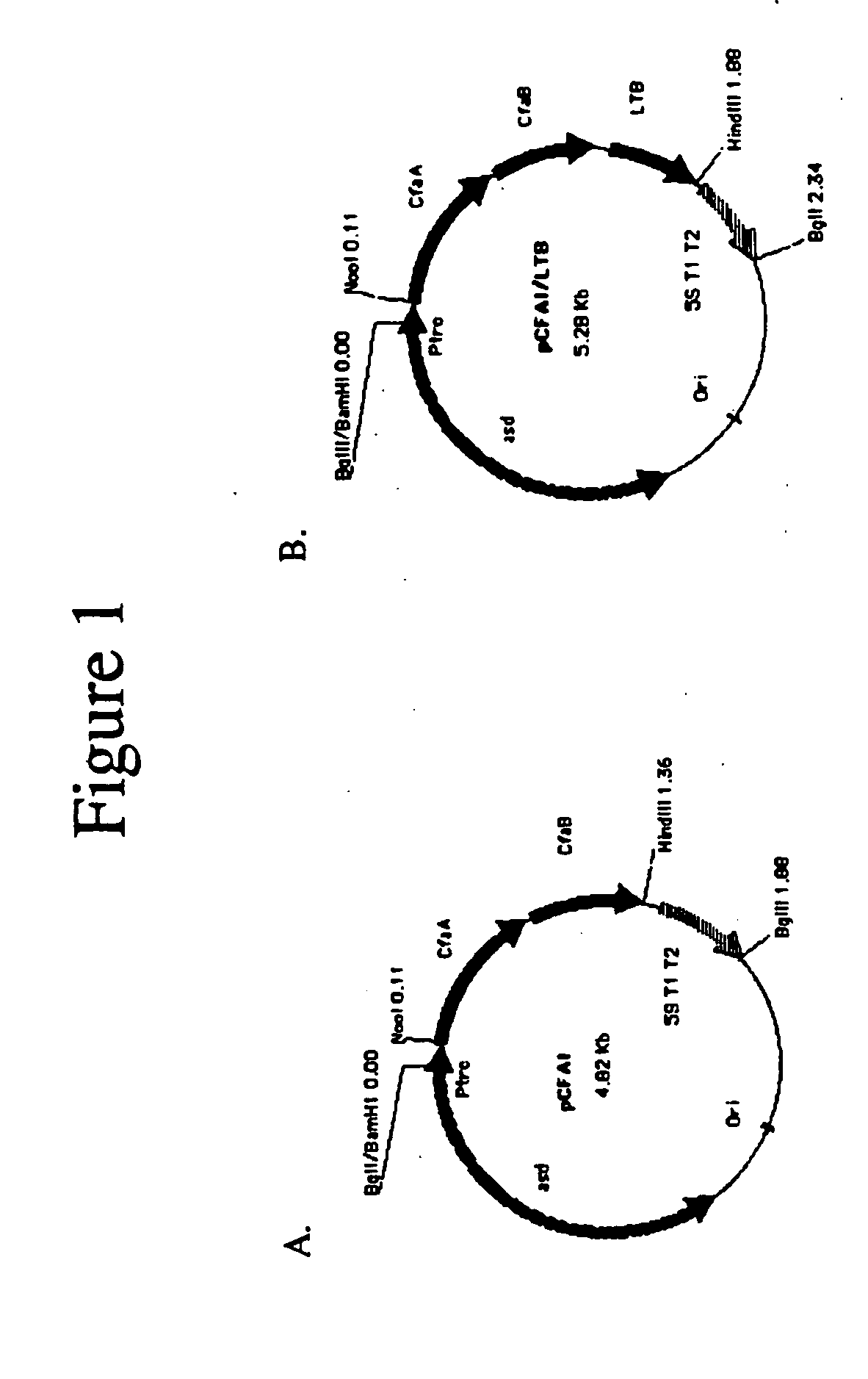
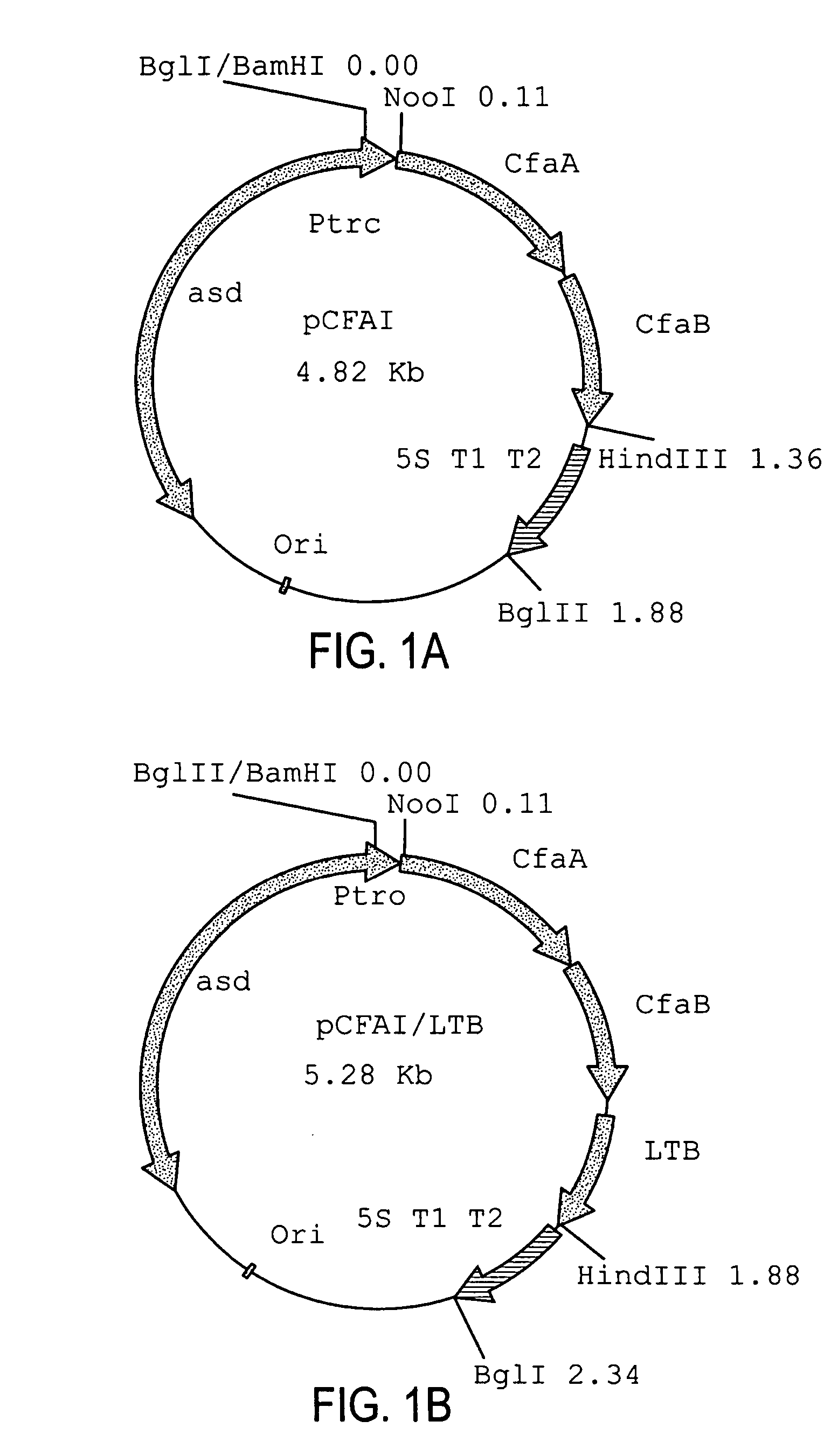
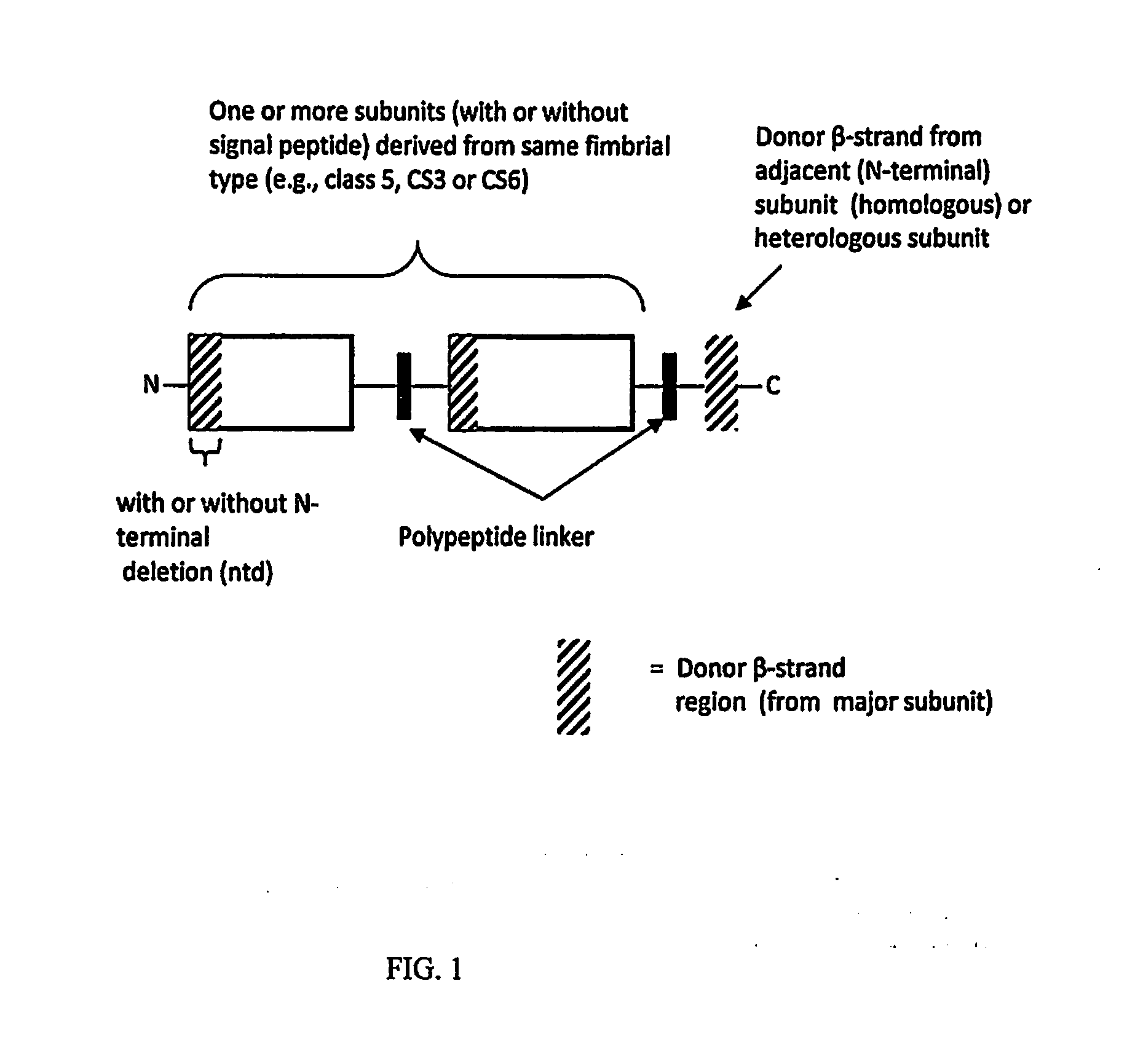
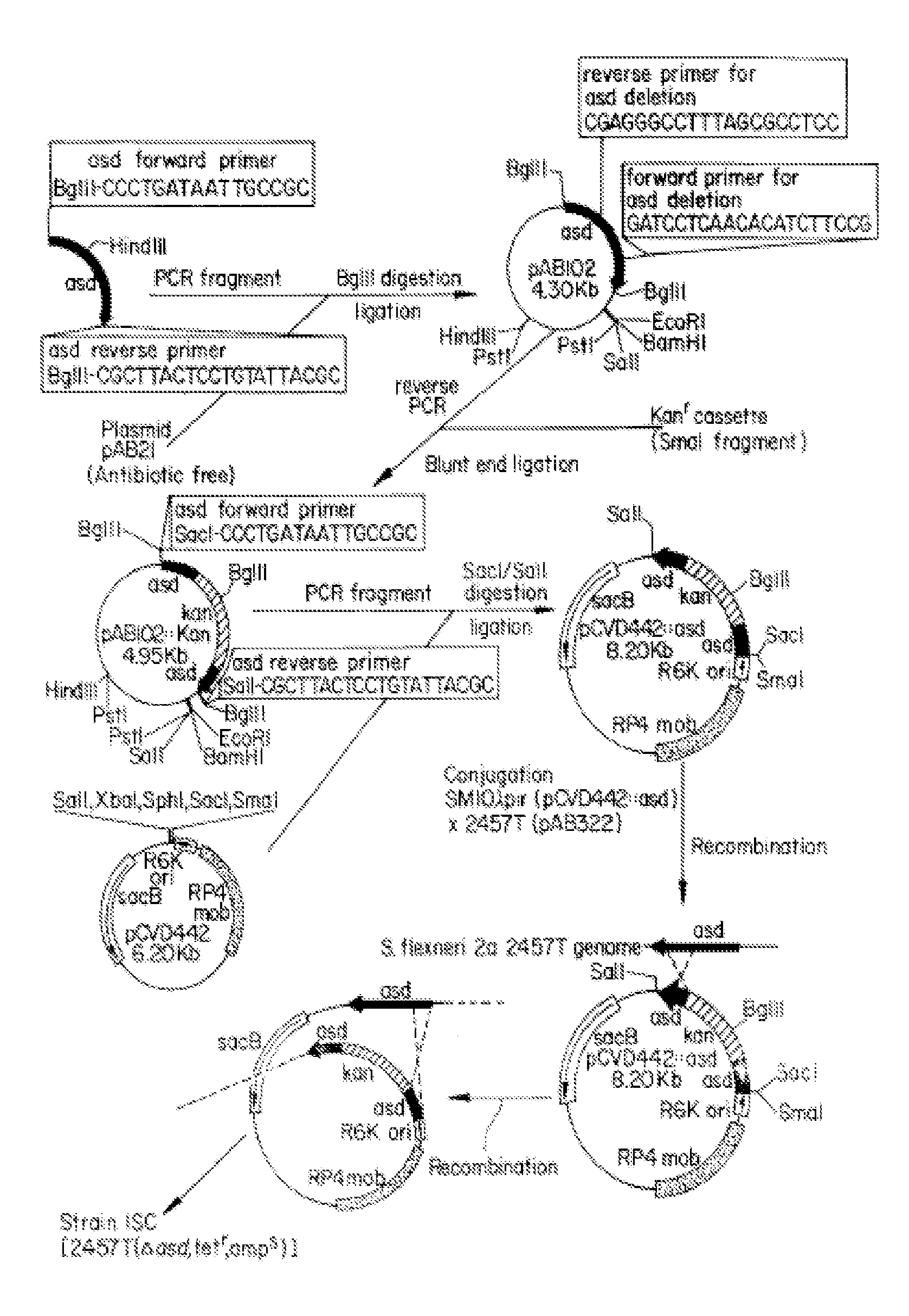
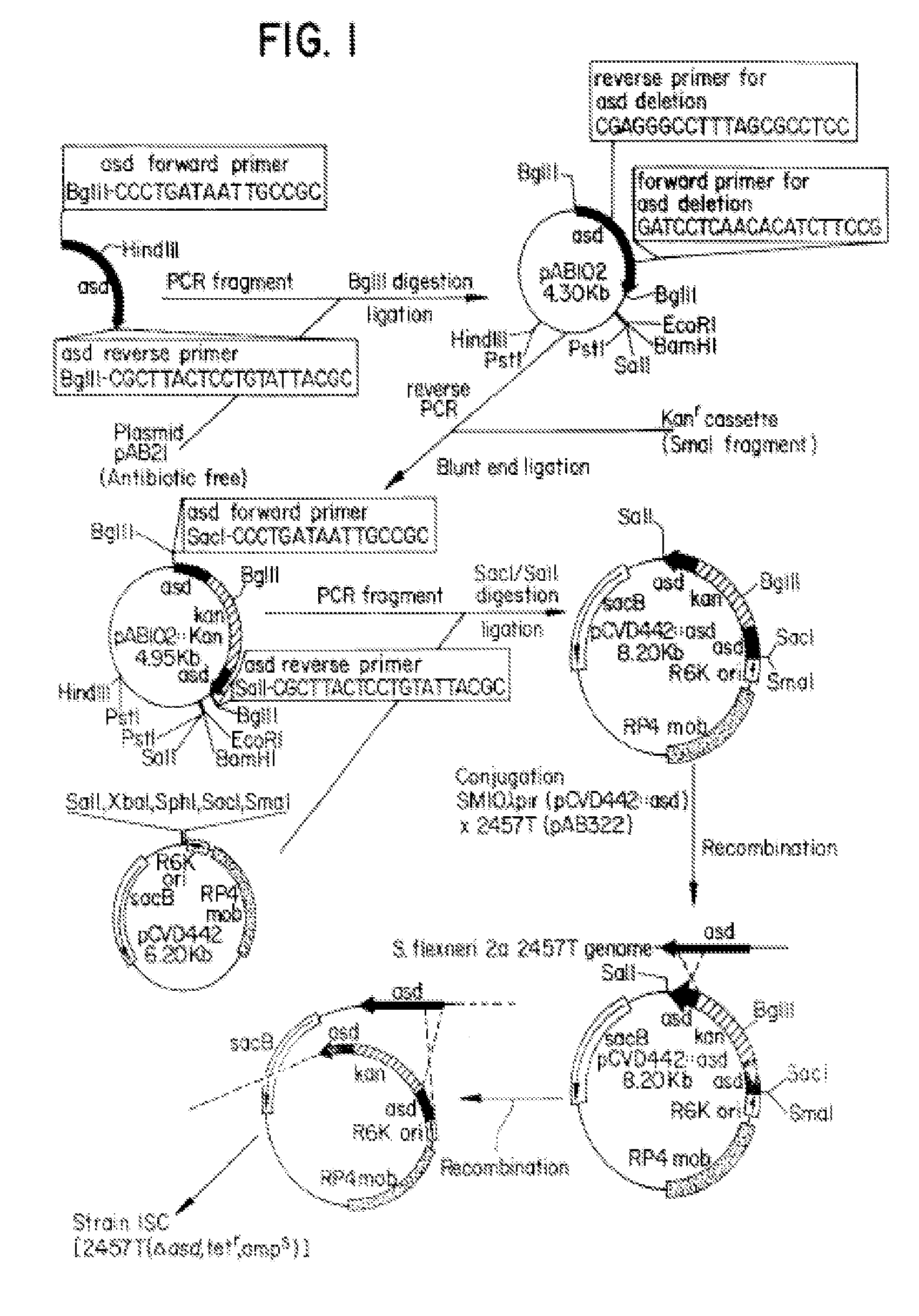
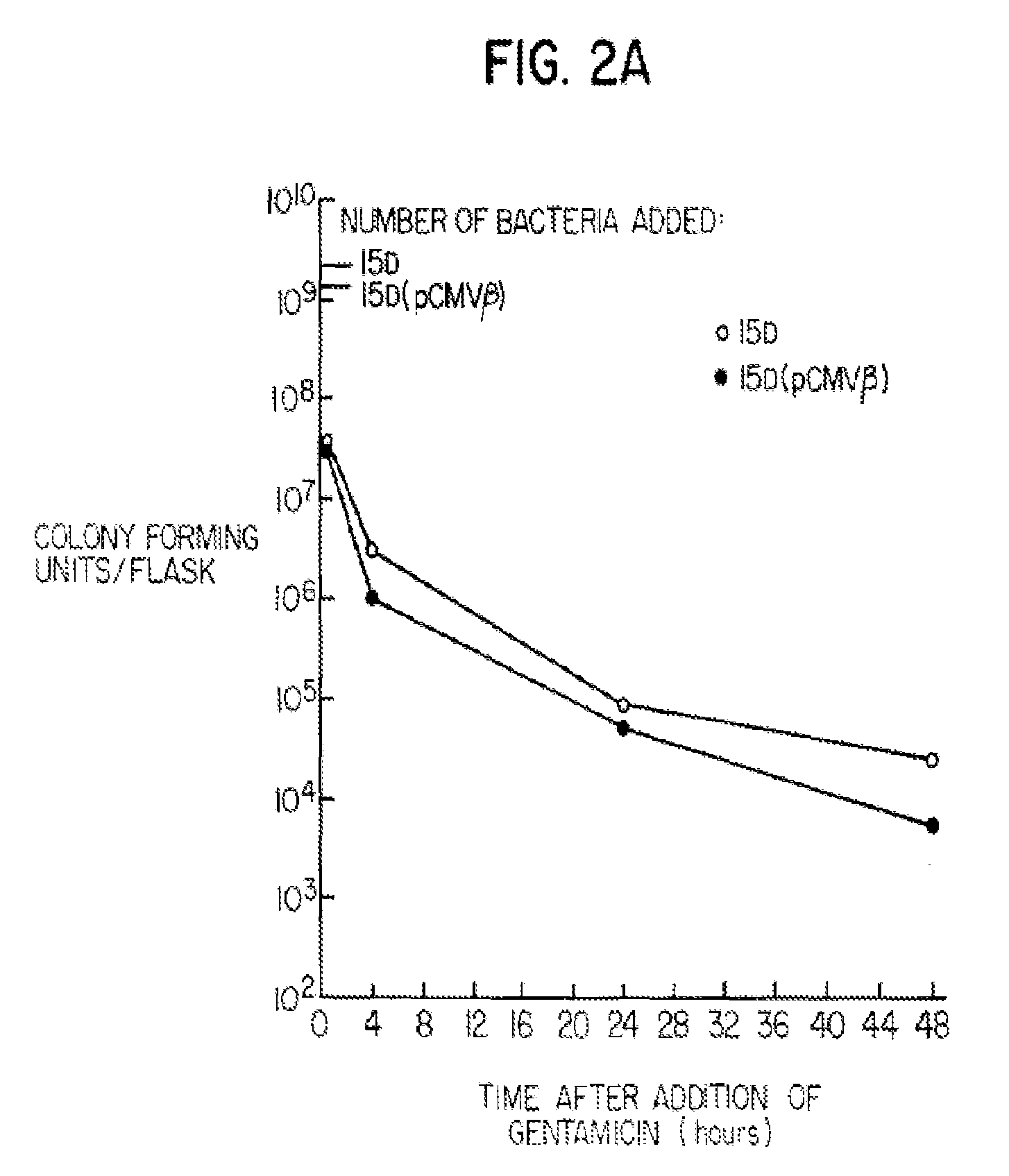
Construction of live attenuated Shigella vaccine strains that express CFA/I antigens (cfaB and CfaE) and the B subunit of heat-labile enterotoxin (LTB) from enterotoxigenic E.coli
ActiveUS20070237791A1Reduce intrusionBacterial antigen ingredientsBacteriaHeterologousMucosal Immune Responses
With the goal of creating a combination vaccine against Shigella and other diarrheal pathogens we have constructed a prototype vaccine strain of Shigella flexneri 2a (SC608) that can serve as a vector for the expression and delivery of heterologous antigens to the mucosal immune system. SC608 is an asd derivative of SC602, a well-characterized vaccine strain, which has recently undergone several phase 1 and 2 trials for safety and immunogenicity. Using non-antibiotic asd-based plasmids, we have created novel constructs for the expression of antigens from enterotoxigenic E. coli (ETEC), including CFA / I (CfaB and CfaE) and the B-subunit from heat-labile enterotoxin (LTB) in Shigella vaccine strain SC608. Heterologous protein expression levels and cellular localization are critical to immune recognition and have been verified by immunoblot analysis. Following intranasal immunization (SC608(CFAI) and SC608(CFAI / LTB) of guinea pigs, serum IgG and IgA immune responses to both the Shigella LPS and ETEC antigens can be detected by ELISA. In addition, ELISPOT analysis for ASCs from cervical lymph nodes and spleen showed similar responses. All vaccine strains conferred high levels of protection against challenge with wild-type S. flexneri 2a using the Sereny test. Furthermore, serum from guinea pigs immunized with SC608 expressing CfaB and LTB contained antibodies capable of neutralizing the cytological affects of heat-labile toxin (HLT) on Chinese Hamster Ovary (CHO) cells. These initial experiments demonstrate the validity of a multivalent invasive Shigella strain that can serve as a vector for the delivery of pathogen-derived antigens.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
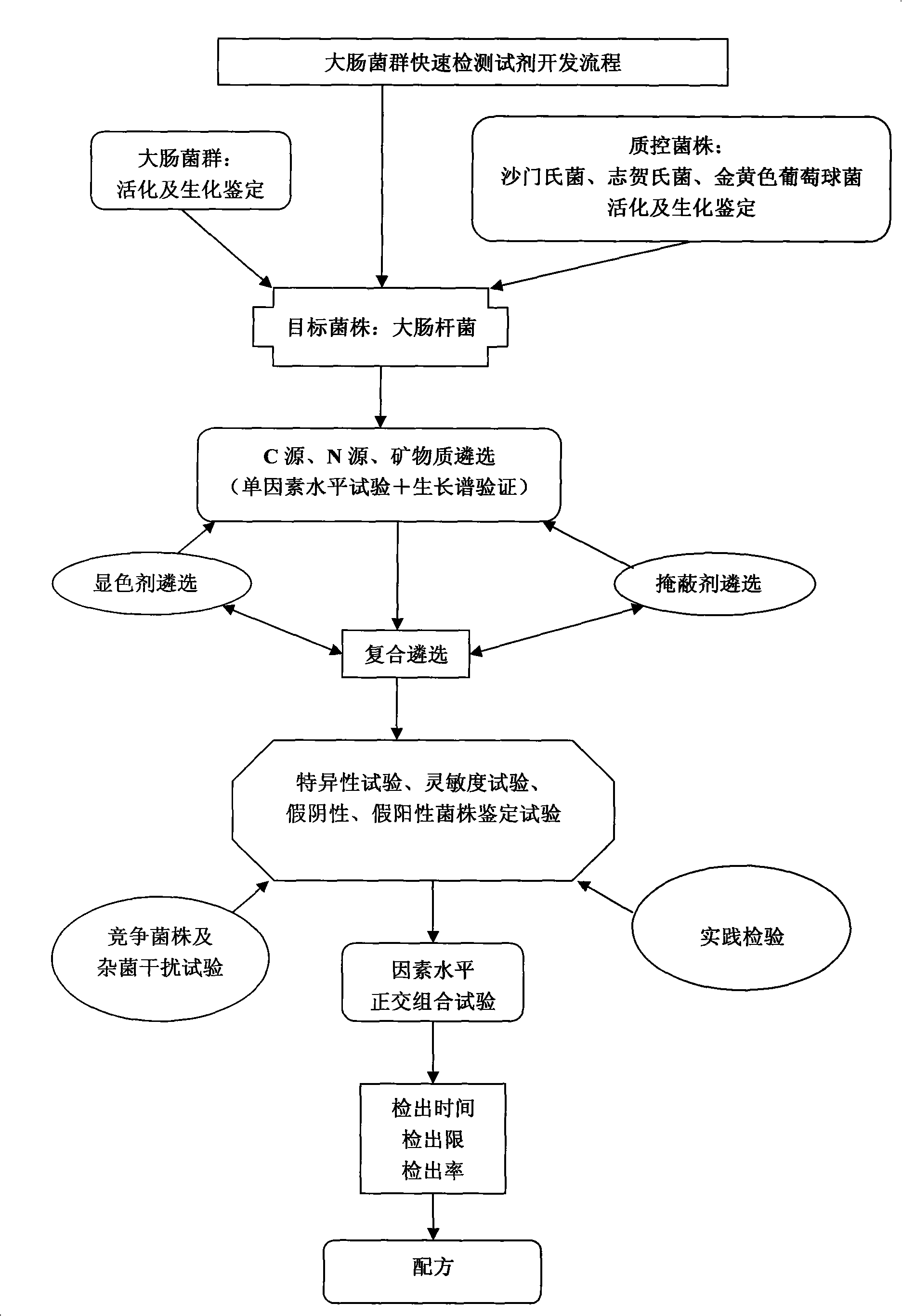
Composite quick colour-developing examination and check agent for coliform group bacteria, researching and developing flow scheme thereof
InactiveCN101294190AGrowth inhibitionMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementEscherichia coliNutrition
The invention relates to a process for the research and development of a colon-bacillus composite and speedy chromogenic identification reagent. The process is characterized in that: 1. First, the quality control method is determined through activation identification and biochemical identification of coliform groups, salmonella, shigella and staphylococcus aureus; 2. Basic nutrients are selected according to the needs of target strains; 3. It is verified by the auxanogram method that proper nutrition environments, carbon sources, nitrogen sources, minerals, microelements, nutrilits, etc. are all necessary for the growth and breeding of microbes; without any one thereof, the microbes can not grow, metabolize and breed normally; 4. A visualization reagent, a masking reagent and a synergistic agent are selected; 5. Composite selection is conducted; 6. The feasibility of the composite selection formula is verified through tests, verifications and examinations on the indexes of a speedy chromogenic substrate, including the specificity test, the sensitivity test, the real sample inspection, the false negative and false positive strain identification, the stability test, the repeatability test, the competing strain and mixed bacterium interference test, the detection time and detection rate tests. 7. The most suitable formula is selected through a factor level orthogonal combination test.
Owner:姚毓才
Gene chip for detecting 11 types of common infectious diarrheal disease pathogen and application thereof
InactiveCN103911443AHigh detection throughputStrong specificityMicrobiological testing/measurementAgainst vector-borne diseasesEscherichia coliAeromonas
The invention relates to a gene chip and an application, which belongs to the biological detection field. The invention designs a set of detection probes by aiming at 11 types of common infectious diarrheal disease pathogen microorganism in clinic, and the gene chip containing the set of the probe. The detection probes comprises a vibrio parahaemolyticus probe, a vibrio vulnificus, a vibrio cholera probe, a vibrio alginolyticus probe, a vibrio furnissii probe, a shigella probe, an escherichia coli probe, an aeromonas probe, a salmonella probe, a campylobacteria probe, and a proteusbacillus vulgaris probe; the above mentioned probe sequence is shown as SEQ ID No: 1-117. The gene chip and probe can detect 11 types of common infectious diarrheal disease pathogen, the detection flux is high, the specialty is strong, the sensitivity is high, and the detection is rapid and effective.
Owner:YANTAI YUHUANGDING HOSPITAL
Salmonella and shigella joint detection kit and detection method thereof
ActiveCN101864483AHigh yieldHigh yield is high; 4. detection kit sensitivity of the present inventionMicrobiological testing/measurementAgainst vector-borne diseasesPositive controlBetaine
The invention relates to a salmonella and shigella joint detection kit and a detection method thereof. The kit of the invention comprises Bst DNA polymerase, buffer solution, dNTPs, betaine, magnesium sulfate, development solution, stabilizing solution and positive control; and the kit also comprises two pairs of primers, namely inner primers FIP / BIP and outer primers F3 / B3 which take salmonella AgfA genes and shigella ipaH genes as target genes and are designed based on loop-mediated thermostatic amplification technology. The salmonella and shigella detection kit has the advantages of more comprehensive detection effect, high specificity and low omission factor, and is suitable for quickly detecting salmonella and shigella in physical examination of food employees.
Owner:GUANGZHOU HUAFENG BIOTECH +1
Gene chip for detecting six kinds of diarrhea pathogens and its prepn process and kit
InactiveCN101045944AAccurate acquisitionNo pollution in the processMicrobiological testing/measurementAgainst vector-borne diseasesVibrio parahaemolyticusDiarrhea
The present invention relates to one kind of gene chip for detecting six kinds of diarrhea pathogens, and the gene chip includes solid carrier and oligonucleotide probe fixed on the carrier. The oligonucleotide probe includes DNA or cDNA segments selected from nucleotide sequences corresponding to the genomes of Shigella, haemorrhagic colibacillus, invasive colibacillus, Vibrio parahaemolyticus, Vibrio cholreae and salmonella. The said chip together with sample treating reagent, hybridizing reagent, color reagent and the specification constitutes the detection kit. The present invention has high detection efficiency and high detection accuracy.
Owner:IPE BIOTECHNOLOGY CO LTD
Detection genetic chip and detection kit for infectious diarrhea
InactiveCN102140507ALow costImprove throughputMicrobiological testing/measurementAgainst vector-borne diseasesBacteroidesEscherichia coli
The invention provides a genetic chip and a detection kit related to major pathogenic microorganisms causing infectious diarrhea of human beings, which are mainly specific to 13 types of bacteria including lapactic bacillus coli / shigella, salmonella, comma bacillus, vibrio parahemolyticus, aeromona hydrophila, plesiomonas, virio hollisae, vibrio fluvialis, vibrio mimicus, Wauter's strains, vibriodamsela and vibrio furnissii. The genetic chip comprises a solid-phase carrier and an oligonucleotide probe fixed on the solid-phase carrier, wherein the oligonucleotide probe comprises DNA (Deoxyribonucleic Acid) fragments selected from genes including a gyrB gene, an ITS gene, a dnaJ gene, a toxR gene and the like with remarkable biological evolution advantages or complementary DNA fragments. By adopting the genetic chip and the detection kit provided by the invention, major pathogenic microorganisms causing infectious diarrhea of human beings can be detected. The genetic chip and the detection kit have the characteristics of easiness and convenience for operation, high flux, high accuracy, high repeatability and the like, and can be applied to clinical detection of inspection departments in hospitals.
Owner:NANKAI UNIV
Gene chip and applications thereof in detection of aquatic pathogenic microorganism
InactiveCN102154473AOvercome time-consuming and labor-intensive defectsHigh detection sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesConserved sequenceSignal on
The invention relates to a gene chip in the detection of aquatic pathogenic microorganism, a preparation method and applications thereof in the detection of aquatic pathogenic microorganism. A conserved sequence selecting from a target pathogenic microorganism gene is fixed on a solid-phase carrier as an oligonucleotide probe, in the proper hybridization condition, a marked sample to be detected is added for reaction, and the corresponding aquatic pathogenic microorganism can be identified according to a probe display signal on the gene chip. The gene chip can fast detect the aquatic pathogenic microorganism such as aeromonas hydrophila, edwardsiella tarda, staphylococcus aureus, vibrio parahaemolyticus, listeria monocytogenes, salmonella typhimurium, shigella and the like, thus being high in detection sensitivity, and strong in specificity and greatly shortening the detection period.
Owner:HANGZHOU ACAD OF AGRI SCI
Composite gene chip for food-borne pathogenic bacteria detection
InactiveCN101397586AExplicitly excludeClear and distinctMicrobiological testing/measurementAgainst vector-borne diseasesEscherichia coliFood borne
The invention relates to a composite gene chip for detecting food pathogenic microbes, which comprises eight parallel sub-arrays constructed by detection probe groups for detecting each microbe in the eight food pathogenic microbes, blank lines are respectively arranged among the eight sub-arrays to be as intervals, thus constructing a big array; the eight food pathogenic microbes are listeria monocytogenes, comma bacillus, vibrio parahaemolyticus, salmonella, shigella, staphylococcus aureus, campylobacter jejuni and escherichia coli. The gene chip can clearly and accurately detect the species of eight target food pathogenic microbes, and also can definitely exclude other off-target microbe samples out of the eight target food pathogenic microbes. Each food pathogenic microbe detection sub-array on the chip can clearly and definitely distinguish off-target microbe samples from target microbe samples.
Owner:广东省疾病预防控制中心
Combined enteropathogen recombinant construct
ActiveUS20150258201A1Avoid undesirable associationShorten the lengthBacterial antigen ingredientsPeptide preparation methodsAntigenBacteroides
The inventive subject matter relates to a construct comprising antigens derived from multiple enterobacteria including Campylobacter jejuni capsule polysaccharide polymer, enterotoxigenic Escherichia coli recombinant polypeptide construct and lipopolysaccharide from Shigella spp. The subject invention also relates to a method of inducing an immune response utilizing the inventive composition.
Owner:UNIVERSITY OF GUELPH +1
Method for detecting food-derived pathogenic enterobacteria by composite fluorescence PCR technique
InactiveCN101113471AStrong specificityAccurate identificationMicrobiological testing/measurementBiological testingEscherichia coliBacteroides
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
Primer for detection of shigella and detection method
InactiveCN101845493AQuick checkEasy to operateMicrobiological testing/measurementMicroorganism based processesShigella dysenteriaeMicrobiology
The invention discloses a primer for detection of shigella and a detection method. The sequence of the primer for the detection of the shigella is expressed as SEQ ID NO: 1-4. The detection method for the shigella comprises the following steps of: (1) extracting DNA serving as a template; (2) adopting a loop-mediated isothermal amplification reaction system which comprises 2muL of template, 2muL of primer, 1muL of Bst DNA and 7.5 to 12.5muL of amplification reaction solution, adding water into the reaction system to obtain 25muL of reaction system, performing thermostatic reaction for 1 to 1.5 hours at the temperature of between 63 and 65 DEG C, then placing the reaction system at the temperature of 80 DEG C for 2 to 10 minutes, and terminating the reaction; and (3) observing the results, namely observing a detection tube by using naked eyes, wherein the result is positive shigella when turbid is produced, and the result is negative when no turbid is produced. The method for detecting the shigella by using the primer to perform the loop-mediated isothermal amplification reaction does not need special equipment, can quickly detect shigella dysenteriae genes in a detection sample, and has the advantages of specificity, high sensitivity and simple identification method.
Owner:SOUTH CHINA AGRI UNIV
LAMP kit for rapid detection of Shigella
InactiveCN102851382AThe detection process is fastStrong specificityMicrobiological testing/measurementMicroorganism based processesSerodiagnosesBetaine
The invention discloses an LAMP (loop-mediated isothermal amplification) kit for rapid detection of Shigella. The kit is composed of an LAMP reaction solution, a standard positive template, and a negative quality control standard. The LAMP reaction solution contains a Bst DNA polymerase large fragment, primers, an LAMP10*buffer, a dNTPs solution, an MgSO4 solution and betaine. The primers include forward and reverse primers. The kit disclosed in the invention has the advantages of good specificity, high sensitivity, rapidness and convenience, high repeatability, and judgeable results obtained by naked eyes, etc., can perform rapid qualitative detection on Shigella in industrial food, and can be used to replace the continuously used traditional culture method and the serological diagnostic method.
Owner:WUHAN ZHENFU PHARMA CO LTD
Shigella gene quick diagnosis kit based on loop-mediated equal-temperature amplification technology
InactiveCN101008036ARapid expansionHigh yieldMicrobiological testing/measurementBacillary dysenteryBiology
The invention relates to rapid secrebil diagnosis kit for bacillary dysentery based on mediated isothermal amplification technology, and belongs to biological detection agent field. The kit comprises a set of primer, DNA polyase, reacting liquid, sample pretreating liquid, coloured solution and positive contrast liquid; said a set of promer comprises two pair of primers, and the squence is: outer primer1: ACATGAAGAGCAYGCCAACA, and Y stands for T or C; outer primer 2: TCCTCACAGCTCTCAGTGG, inner primer 1: CGGAATCCGGAGGTATTGCGTGTTTTCCTTTTCCGCGTTCCTTGA, inner primer2: GGTCGCTGCATGGCTGGAAATTTTGCAGCAACAGCGAAAGACT, and the primers comprise 2 M inner primer and 0. 25 M outer primer. The kit needs no special agent and device, can fast detect bacillary dysentery gene, and is characterized by high speciality, high sensitivity, simple determination method and high correcting rate.
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN
Gene chip for detecting important pathogenic bacteria in aquatic product and kit thereof
InactiveCN102311993AImprove accuracyGood repeatabilityMicrobiological testing/measurementAgainst vector-borne diseasesBacillus cereusStaphylococcus aureus
The invention relates to a gene chip for detecting important pathogenic bacteria in an aquatic product and a kit thereof. The gene chip comprises a solid phase carrier and an oligonucleotide probe; the oligonucleotide probe comprises one or more of the following nucleotide sequences: (1) DNA (Deoxyribonucleic Acid) sequences selected from proteus mirabilis 1, proteus vulgaris, salmonella, vibrio parahaemolyticus, vibrio cholerae, Listeria monocytogenes, staphylococcus aureus, streptococcus pyogenes, vibrio vulnificus, bacillus cereus cluster, Shigella and pathogenic Y. enterocolitica ail gene; (2) complementary DNA sequences of the DNA sequences selected in the (1); (3) complementary RNA (Ribonucleic Acid) sequences of the DNA sequences in the (1) or (2). The kit comprises the gene chip. The gene chip and the kit can be used for detecting the important pathogenic bacteria in an aquatic product, are convenient to operate, high in precision and strong in repeatability.
Owner:TIANJIN BIOCHIP TECH CO LTD
Primer combination for detecting infectious diarrhea pathogen and kit thereof
ActiveCN106916906AQuick checkAccurate detectionMicrobiological testing/measurementAgainst vector-borne diseasesRotavirus RNARotavirus
The invention relates to a primer combination for detecting infectious diarrhea pathogen. The primer combination comprises at least one combination of the following combinations: a rotavirus primer combination, an enteric adenovirus primer combination, a Norovirus primer combination, a salmonella primer combination, a Shigella primer combination and a campylobacter jejuni primer combination. The invention also relates to a kit containing the primer combination. The kit also comprises a primer-coating micro-fluidic chip, an isothermal amplification reaction liquid, an isothermal amplification enzyme solution, and a negative control. The invention also relates to a detection method by using the above primer combination. The detection method comprises steps as follows: coating of the primer combination, nucleic acid extraction of a sample to be detected, LAMP reaction and result interpretation. The kit provided by the invention can detect infectious diarrhea pathogen rapidly and accurately within one hour, and is also of great significance for rapid assisted guide treatment and drug use. The multi-index detection also can be used in regional epidemiological investigation and epidemic surveillance so as to study epidemic situation of infectious diarrhea in China.
Owner:SHANGHAI IGENETEC DIAGNOSTICS CO LTD
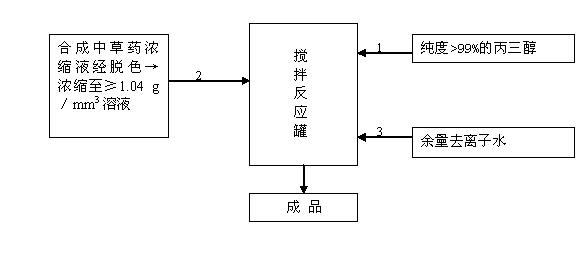
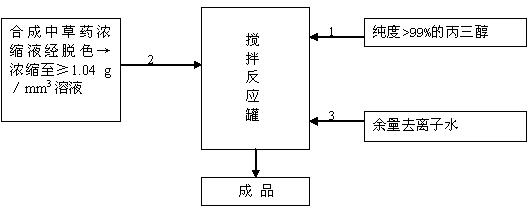
Organic dairy cow breast disinfectant and preparation method thereof
InactiveCN102657801AStrong killing effectPrevent drynessBiocideAntisepticsEscherichia coliDisinfectant
The invention relates to a dairy cow breast disinfectant, and belongs to the fields of biomedicine and medicaments for agriculture and livestock. The organic dairy cow breast disinfectant is characterized by comprising the following raw materials in percentage by mass according to the formula: 0.25 to 1 percent of 99 percent glycerol, 0.25 to 1 percent of synthetic Chinese medicinal herb concentrate, and the balance of deionized water. The Chinese medicinal herb concentrate is prepared from extracts of honeysuckle, indigowoad leaf, Chinese violet, muskroot-like semiaquilegia root, atractylodes rhizome, folium artemisiae argyi, phyllanthus ruinaria, dandelion, sophora flavescens, glabrous greenbrier rhizome, fructus cnidii, patrinia and groundsel by cleaning, extraction, re-extraction of primary residue, synthesis, decolorization and concentration, and the density of the concentrate is more than or equal to 1.04g / mm<3>. The disinfectant is used for diminishing dairy cow breast inflammation caused by invasion of multiple microbes such as candida albicans, escherichia coli, streptococcus, microsporum, salmonella and shigella and controlling invasion of pathogenic microbes on breasts and mammary glands of dairy cows.
Owner:SHANGHAI UNIV +1
Microorganisms as carriers of nucleotide sequences coding for antigens and protein toxins, process of manufacturing and uses thereof
ActiveUS8669091B2Efficient tumor therapyEnhance immune responseAntibacterial agentsBacteriaAntigenTransport system
A Escherichia, Salmonella, Yersinia, Vibrio, Listeria, Shigella, or Pseudomonas bacterium that has the following components: (I) a polynucleotide encoding a heterologous antigenic determinant that induces a CTL response against a tumor cell; (II) a polynucleotide encoding a heterologous protein toxin or toxin subunit; and (III) (a) a polynucleotide encoding a transport system that expresses the products of (I) and (II) on the outer surface of the bacterium or that secretes products of (I) and (II) from the bacterium; and (IV) a polynucleotide that activates the expression of one or more of (I). (II), and / Or (III) in the bacterium wherein polynucleotides (I), (II), (III) and (IV) are different from each other and polynucleotides (I), (II) and (III) encode proteins that are different from each other.
Owner:SOCIUM THERAPEUTICS INC
Shigella flora/serotype multiplex-PCR (polymerase chain reaction) detection primer set and kit
InactiveCN103451307AShorten detection timeTo achieve the detection effectMicrobiological testing/measurementAgainst vector-borne diseasesShigella sonneiElectrophoresis
The invention discloses a Shigella flora / serotype multiplex-PCR (polymerase chain reaction) detection primer set and kit. The detection primer set is composed of primer pairs for detecting Shigella sonnei, type-1-5 Shigella flexneri, type-I Shigella dysenteriae, type-6 Shigella flexneri, and Shigella. According to the invention, a multiplex-PCR detection method based on a common PCR platform is built; according to the method, multiplex-PCR reaction is carried out on total DNA of bacteria extracted from a sample to be detected in a same reaction system by using the primer pairs obtained through analysis and design, and through electrophoretic analysis on a reaction product, whether the sample is Shigella can be determined and Shigella flora / serotype can be judged.
Owner:北京卓诚惠生生物科技股份有限公司
Traditional Chinese medicine preparation with bacteriostasis effect and production method thereof
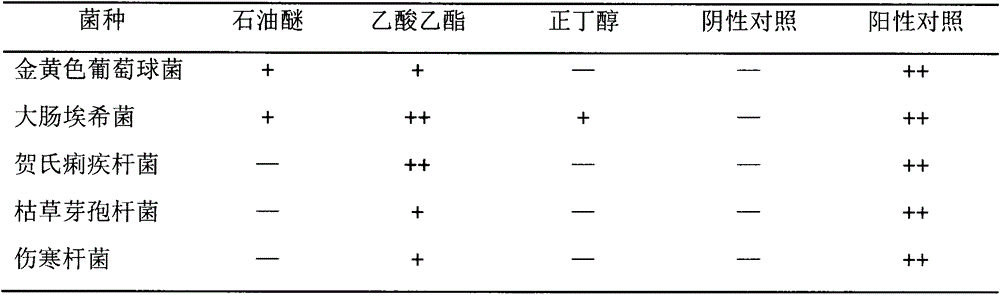
The invention belongs to the field of traditional Chinese medicine, and relates to a traditional Chinese medicine preparation with a bacteriostasis effect, in particular to a traditional Chinese medicine preparation prepared from extracts of asian tetracera roots or leaves (roots of Tetracera asiatica(Lour.)Hoogland). Research results show that the traditional Chinese medicine preparation has a bacteriostasis activity effect on Shigella castellani, escherichia coli, staphylococcus aureus, bacillus subtilis and typhoid bacillus; good bacteriostasis and anti-inflammation effects are shown.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
Method and kit for detecting Shigella and ipaH pathogenicity island thereof
ActiveCN101235413ASensitive diagnosisRapid diagnosisMicrobiological testing/measurementFluorescence/phosphorescenceBacteroidesFluorescence
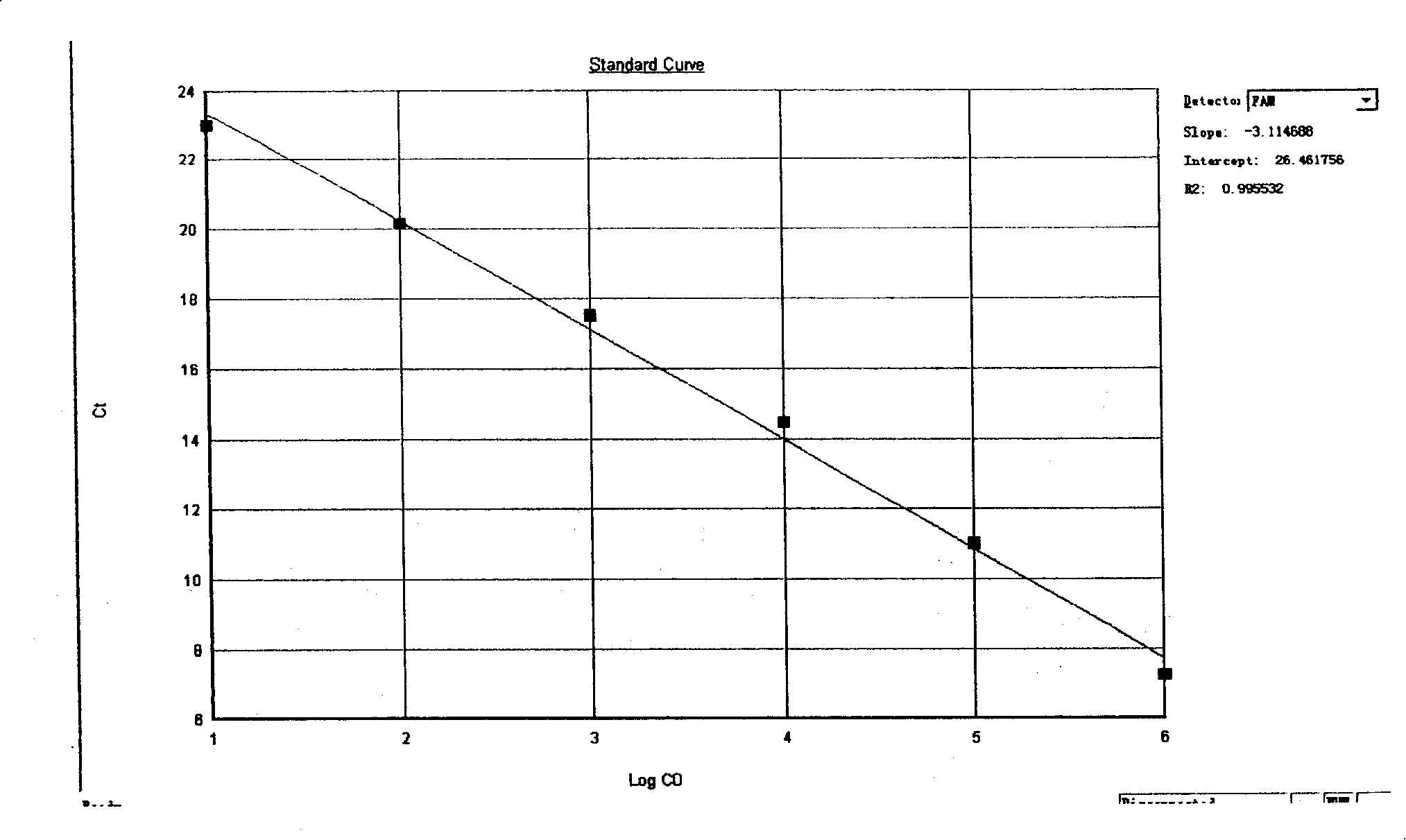
The invention relates to a method and a reagent for detecting the existence of shigella bacteria genus bacteria and ipaH pathogenicity island (PAI) of the shigella in clinical samples and in particular relates to the method and the eagent kit for detecting the shigella with real-time fluorescence quantitative polymerase chain reaction technique.
Owner:DAAN GENE CO LTD
Bacterial delivery system
We describe a bacterial delivery system for the delivery of DNA and antigens into cells. We constructed an attenuated bacterial vector which enters mammalian cells and ruptures delivering functional plasmid DNA and antigens into the cell cytoplasm. This Shigella vector was designed to deliver DNA to colonic surfaces, thus opening the possibility of oral and other mucosal DNA immunization and gene therapy strategies. The attenuated Shigella is also useful as a vaccine for reducing disease symptoms caused by Shigella.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Disinfectant for hog house
The invention discloses a disinfectant for hog house. The disinfectant comprise the following raw materials by weight: 20 to 30 parts of Artemisia argyi, 15 to 20 parts of mozzie buster, 5 to 8 parts of clove, 20 to 30 parts of honeysuckle flower, 10 to 15 parts of common nepenthes, 10 to 15 parts of Chrysanthemum cinerariifolium, 10 to 15 parts of lavender and 15 to 20 parts of geranium. The disinfectant has good sterilization and bacteriostatic effect, can effectively inhibit and kill pathogenic bacteria, fungi and viruses in an environment, exerts inhibitory and killing effect on a variety of parasites, and especially shows good bacteriostatic effect on Escherichia coli, Staphylococcus aureus and Shigella species.
Owner:TIANJIN JIHE TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com