Prussian-blue type sodium ion battery positive electrode material and preparation method therefor
A sodium-ion battery and Prussian blue technology, which is applied in the field of sodium-ion battery cathode materials and its preparation, can solve problems such as capacity loss, and achieve the effects of reducing capacity fading, excellent performance, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Dissolve 0.968g of sodium ferrocyanide decahydrate in 100mL of deionized water to obtain a 20mmol / L sodium ferrocyanide solution.
[0032] 2) Dissolve 0.398g of ferrous chloride tetrahydrate in 100mL of deionized water to obtain a 20mmol / L ferrous chloride solution.
[0033] 3) Dissolve 0.285g of nickel dichloride hexahydrate and 0.159g of ferrous chloride tetrahydrate in 100mL of deionized water to obtain a mixed solution with a total cation concentration of 20mmol / L.
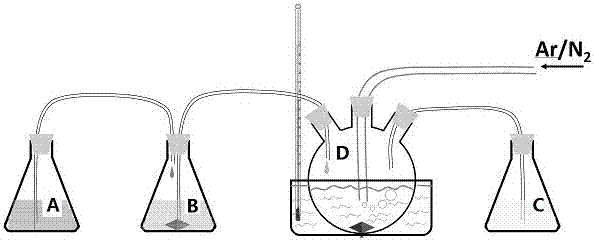
[0034] 4) Use as attached figure 1The shown device carries out co-precipitation reaction, and container A is mixed solution, and in container B is ferrous chloride solution, and container C is sodium ferrocyanide solution, and D is reaction vessel, and deionized water is filled in reaction vessel; A and container B, container B and reaction container, container C and reaction container are all connected by a catheter with a peristaltic pump; wherein, container A is connected at the beginning of the ...
Embodiment 2
[0039] 1) Dissolve 0.242g of sodium ferrocyanide decahydrate in 100mL of deionized water to obtain a 5mmol / L sodium ferrocyanide solution.
[0040] 2) Dissolve 0.099g of ferrous chloride tetrahydrate in 100mL of deionized water to obtain a 5mmol / L ferrous chloride solution.
[0041] 3) Dissolve 0.050 g of manganese dichloride tetrahydrate and 0.050 g of ferrous chloride tetrahydrate in 100 mL of deionized water to obtain a mixed solution with a total cation concentration of 5 mmol / L.
[0042] 4) same as embodiment 1, utilize as attached figure 1 The setup shown performs the co-precipitation reaction. Among them, container A is inserted at the beginning of the reaction. During the reaction process, the reaction vessel was filled with nitrogen gas, the flow rate was 20mL / min, the reaction vessel was heated to 25°C by a magnetic stirrer, and the flow rate of all peristaltic pumps was the same as 10mL / h, until the sodium ferrocyanide solution in vessel C was consumed When finis...
Embodiment 3
[0045] 1) Dissolve 4.84g of sodium ferrocyanide decahydrate in 100mL of deionized water to obtain a 100mmol / L sodium ferrocyanide solution.
[0046] 2) Dissolve 1.99g of ferrous chloride tetrahydrate and 0.5mg of vitamin C in 100mL of deionized water to obtain a 100mmol / L ferrous chloride solution.
[0047] 3) Dissolve 2.380 g of cobalt dichloride hexahydrate in 100 mL of deionized water to obtain a cobalt chloride solution with a total cation concentration of 100 mmol / L.
[0048] 4) same as embodiment 1, utilize as attached figure 1 The setup shown performs the co-precipitation reaction. Wherein, container A is inserted when the sodium ferrocyanide solution balance in container C is 10mL. During the reaction process, the reaction vessel was filled with argon gas at a flow rate of 100mL / min. The reaction vessel was heated to 90°C by a magnetic stirrer, and the flow rate of all peristaltic pumps was the same as 10mL / h. After consumption, turn off all peristaltic pumps and st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com