A kind of side chain type polyimide material and its preparation method and application
A polyimide and side chain technology, applied in the field of organic optical functional materials, can solve the problems of fast decay of electro-optic activity, poor material orientation stability, doping, etc. The method is simple, convenient for chromophore content, reaction The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
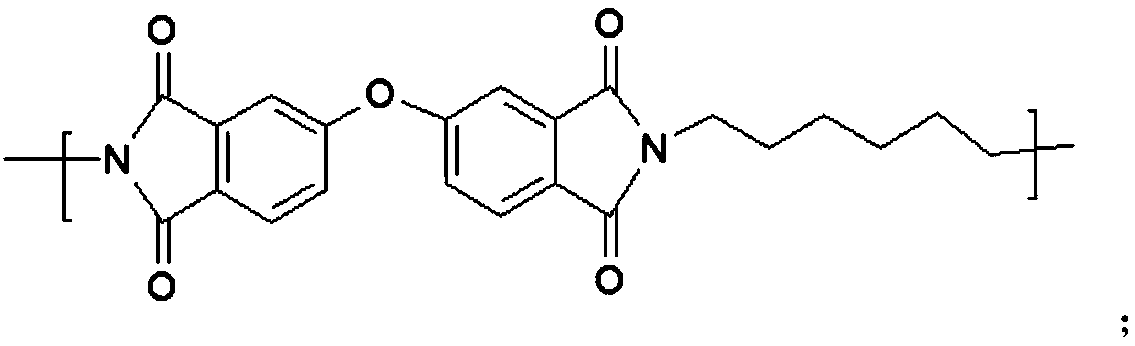
[0051] Synthesis of Hydroxyl-containing Polyimide (PI-OH)
[0052]
[0053] Aromatic dianhydride monomer 3,3',4,4'-diphenyl ether tetracarboxylic anhydride (10mmol), diamine monomer containing hydroxyl functional group 2,3-bis(3-amino-4-hydroxyphenyl ) propane (5mmol) and the aliphatic diamine monomer 1,6-hexanediamine (5mmol) were dissolved in N,N-dimethylacetamide respectively. Mix the above-mentioned dianhydride monomer and diamine monomer solution, and react at room temperature for 48 hours. Then add 10 mL of xylene, heat up to 150°C with azeotropic water, react for 24 hours, and cool to room temperature. Slowly pour the above solution into the mixed solution of ethanol / water, and adjust to weak acidity with dilute hydrochloric acid, precipitate out, and filter with suction. After preliminary drying, the above precipitate was dissolved in a small amount of tetrahydrofuran, slowly dropped into the mixed solution of ethanol / water, and adjusted to weak acidity with dilut...
Embodiment 2
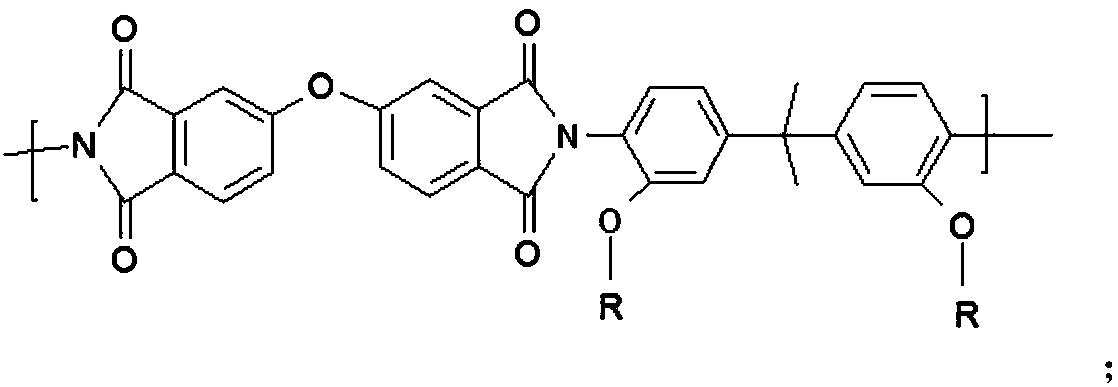
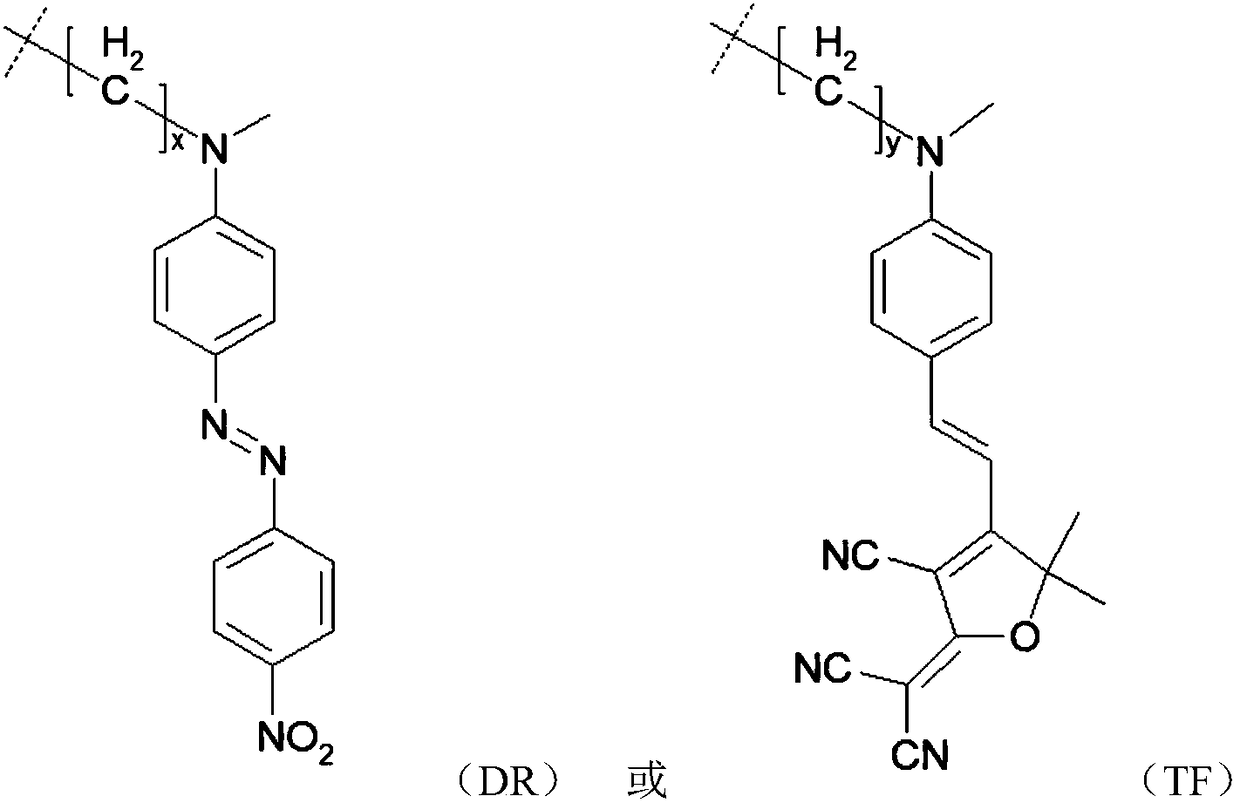
[0055] Synthesis of side-chain electro-optic active polyimide PI-DR
[0056] The polyimide PI-OH (5mmol) that embodiment 1 obtains is dissolved in the mixed solution of 30mL tetrahydrofuran and 20mL dichloromethane, then successively chromophore DR (1mmol) under nitrogen protection, triphenylphosphine (1.2 mmol), diethyl azodicarboxylate (1.2mmol). React at room temperature for 48 hours, slowly drop into the mixed solution of ethanol / water, and adjust to weak acidity with dilute hydrochloric acid, precipitate out, and filter with suction. After dissolving with a small amount of tetrahydrofuran, the process of sedimentation and suction filtration was repeated several times, and after vacuum drying, a purple-red side chain type electro-optic polyimide PI-DR was obtained, wherein m=15, n=58. T g :168℃; Td 5% : 295°C; UV-Vis (tetrahydrofuran): λ max = 470nm.
Embodiment 3
[0058] Synthesis of side-chain electro-optic active polyimide PI-TF
[0059] The polyimide PI-OH (5mmol) that embodiment 1 obtains is dissolved in the mixed solution of 30mL tetrahydrofuran and 20mL dichloromethane, then successively chromogen TF (1.5mmol) under nitrogen protection, triphenylphosphine ( 1.8mmol), diethyl azodicarboxylate (1.8mmol). React at room temperature for 72 hours, slowly drop into the mixed solution of ethanol / water, and adjust to weak acidity with dilute hydrochloric acid, precipitate precipitates, and filter with suction. After dissolving with a small amount of tetrahydrofuran, the process of sedimentation and suction filtration was repeated several times, and after vacuum drying, a purple-red side chain type electro-optical polyimide PI-TF was obtained. T g :162℃; Td 5% : 280°C; UV-Vis (tetrahydrofuran): λ max = 550nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com