Label-free electrochemical sensing detection method used for activity of protein tyrosine phosphatase
A technology of tyrosine phosphatase and tyrosine, which is applied in biochemical equipment and methods, determination/inspection of microorganisms, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 The functional modification of the semiconductor electrode surface
[0034] Since we use a sensor detection method, it is necessary to functionally modify the surface of the indium tin oxide (ITO) electrode. The modifiers that can be used are generally organic compounds with poor conductivity, which may inhibit the electrochemical response of the electronic mediator. We have carried out different degrees of silanization modification on the surface of the electrode, and respectively placed the ITO electrode in a toluene solution containing 1% 3-glycidyl etheroxypropyltrimethoxysilane to react for different times (0.5, 1, 2, 4 , 8, 10, 12, 16, 20, 24 hours). After the reaction is completed, ultrasonically sonicate in toluene and absolute ethanol for 5 minutes respectively, then blow dry with nitrogen gas, and bake in a muffle furnace at 115° C. for 20 hours. X-ray photoelectron spectroscopy (XPS) showed that the surface content of the modifiers increased wit...
Embodiment 2
[0035] Example 2 Covalent immobilization of polypeptide molecular film on the surface of indium tin oxide
[0036] First cut the functionally modified ITO electrode into a size of 2.5cm×0.5cm, wash it in pure water, blow dry with nitrogen, and evenly cover and spread 10 μL of different concentrations of peptide solutions (2.0M phosphate buffer solution, pH 7.4) on the 0.5cm×0.5cm ITO electrode, placed in a humid container at 25°C for 24 hours. After the surface reaction, the electrode was shaken and washed in 20 mM phosphate buffer containing 0.1% Tween-20, 20 mM phosphate buffer containing 150 mM sodium chloride, and 20 mM phosphate buffer for 5 minutes, and dried with nitrogen. Finally, the ITO electrode was placed in a Tris-HCl solution to seal the remaining reaction sites on the electrode surface. Rinse with deionized water and dry with nitrogen gas. XPS and cyclic voltammetry were used to measure the fixed amount of polypeptide and the electrochemical activity of polype...
Embodiment 3
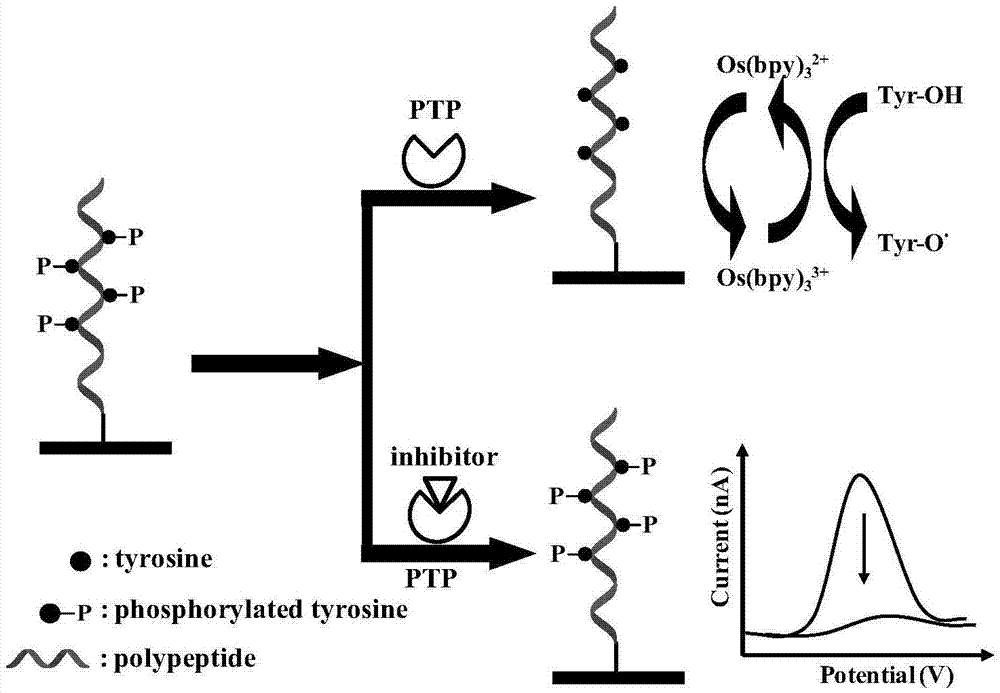
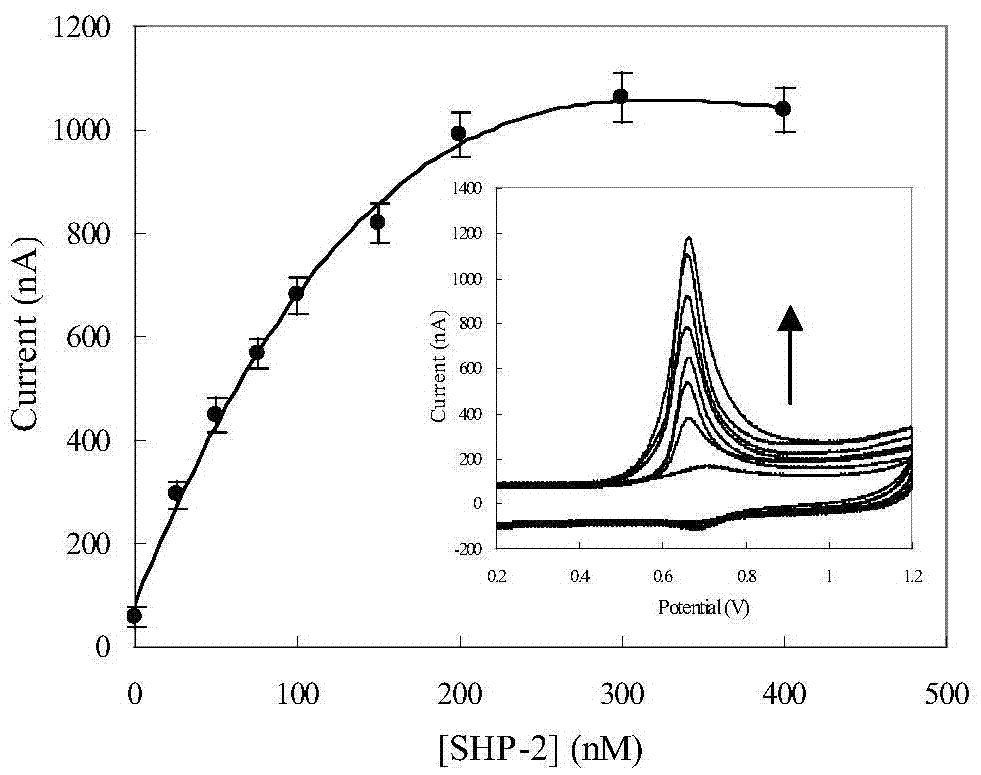
[0037] The detection of embodiment 3 protein tyrosine phosphatase activity
[0038] According to the optimized conditions, place the ITO electrode with the substrate polypeptide immobilized on the surface in the dephosphorylation reaction solution (containing 50mM NaCl, 1mM DTT, 0.05% Tween-20 and PTP or PTP and inhibitor), and react at 37°C for a period of time. time. The electrode was shaken and washed in 20mM phosphate buffer solution containing 0.1% Tween-20, 20mM phosphate buffer solution containing 150mM sodium chloride, and 20mM phosphate buffer solution for 5 minutes, and dried with nitrogen gas for electrochemical detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com