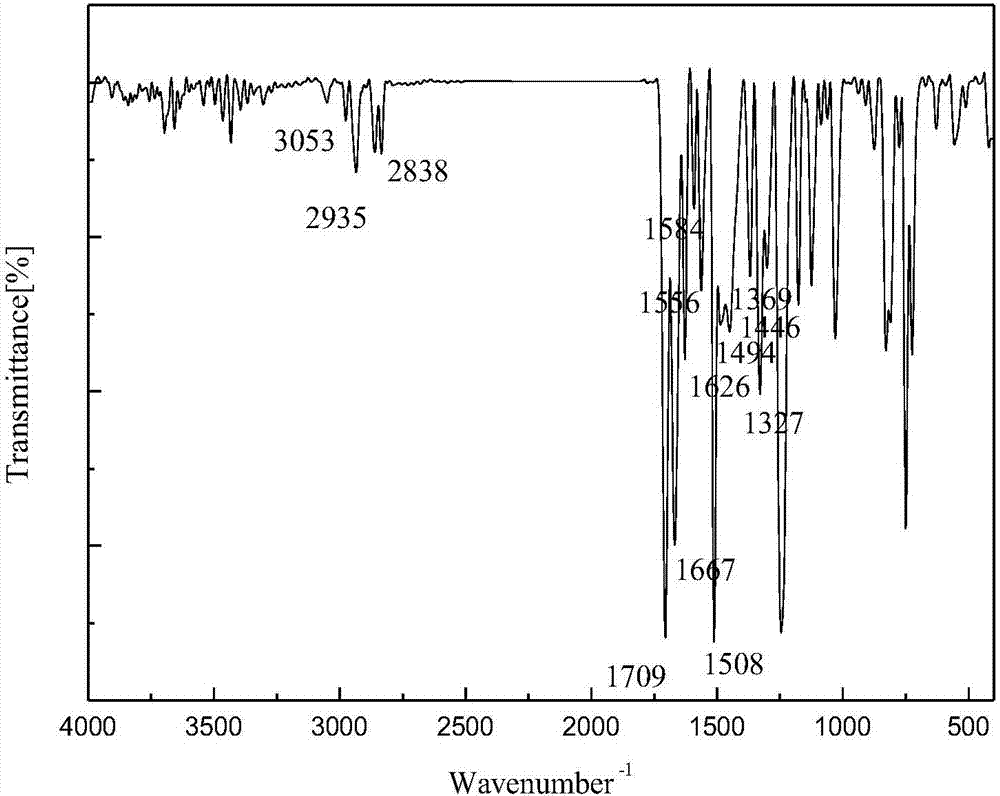
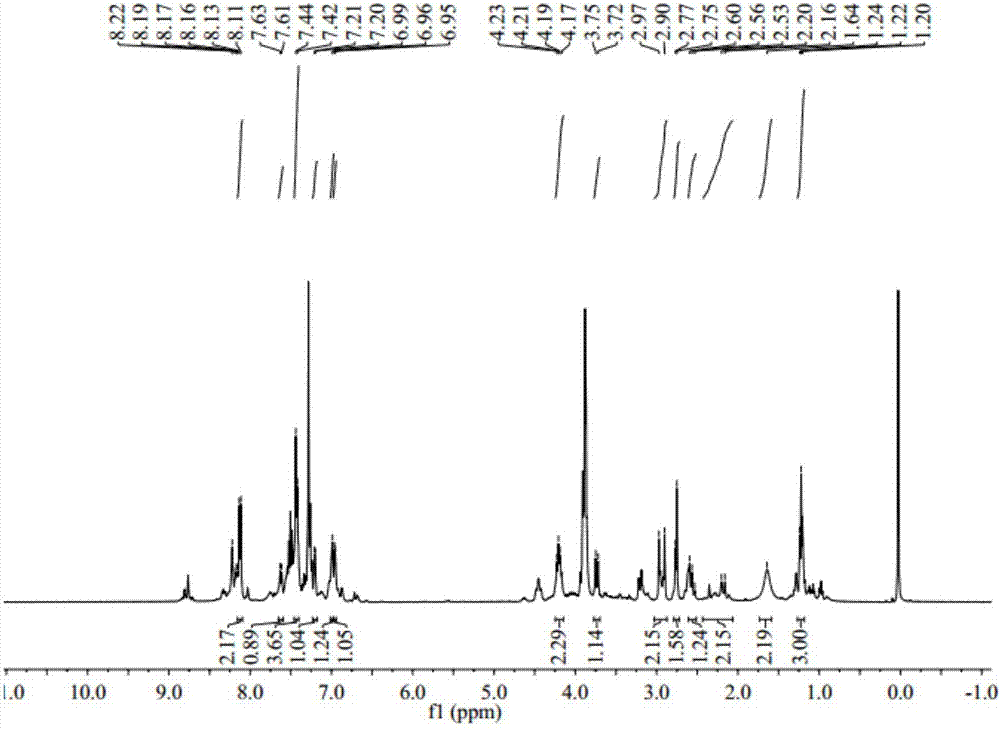
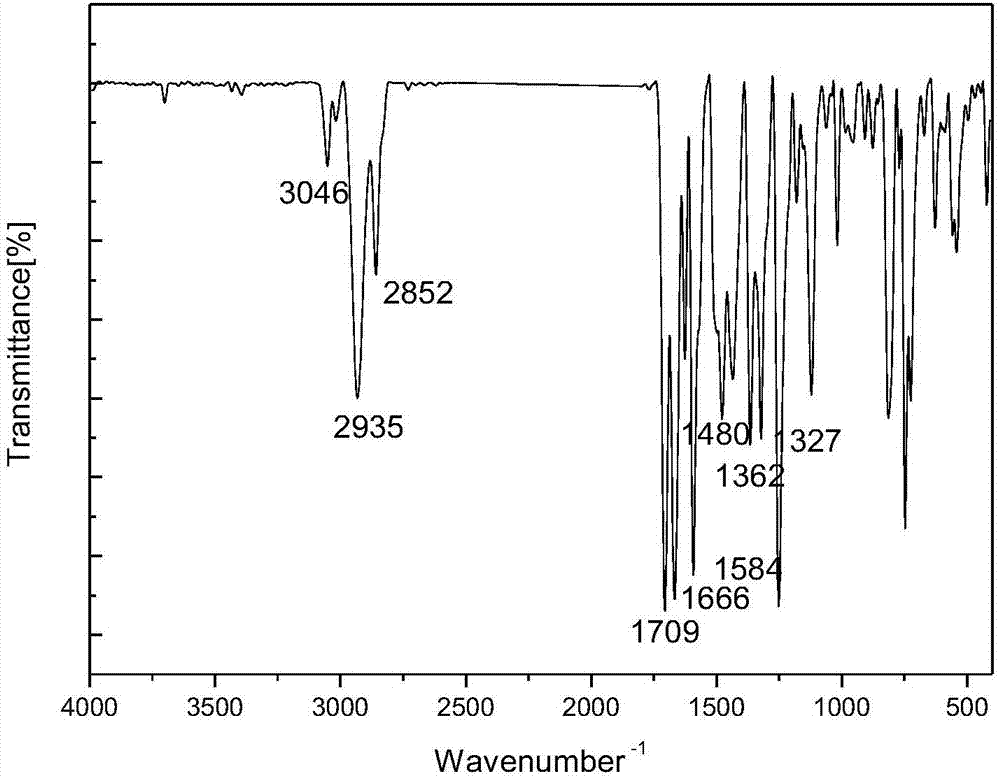
1-(3-N-Substituted-carbazoly)-3-aryl-3-(2-oxocyclohexyl)-acetone and preparation method thereof
A cyclohexanone-based and carbazolyl-based technology, which is applied in the field of 1--3-aryl-3--acetone and its preparation, can solve the problems of strong alkali corrosion equipment and increased cost, and achieve low equipment requirements , fewer by-products, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Sequentially weigh 0.006mol cyclohexanone, 0.006mol NaOH (solid, granular), and 30mL of absolute ethanol, and add them to a dry three-necked flask equipped with a condensing reflux tube and a stirring device, and let it stir at room temperature for 5 minutes. After uniformity, add 0.001mol of 1-(3-N-ethyl-carbazolyl)-3-(p-methoxyphenyl)-propenone, stir and slowly raise the temperature to 80°C, and make it Reflux reaction 40min under the temperature of 70rad / min stirring speed. During the reaction period, the reaction progress was monitored by thin-layer chromatography, and a developer with a volume ratio of V (ethyl acetate): V (petroleum ether) = 1:3 was selected. After the reaction is complete, stop the reaction, let the reaction system drop to room temperature slowly, add 30mL of 3°C cold water, let it stand still, a solid precipitates, filter with suction, wash the filter cake repeatedly until it is neutral, recrystallize it with absolute ethanol, and evaporate it w...
Embodiment 2
[0047] Sequentially weigh 0.006mol cyclohexanone, 0.006mol NaOH (solid, granular), and 30mL of absolute ethanol, and add them to a dry three-necked flask equipped with a condensing reflux tube and a stirring device, and let it stir at room temperature for 5 minutes. After uniformity, add 0.001mol 1-(3-N-ethyl-carbazolyl)-3-(2-thienyl)-propenone, stir and slowly raise the temperature to 70°C, make it at 70°C and Reflux reaction for 30min at a stirring speed of 70rad / min. During the reaction period, the reaction progress was monitored by thin-layer chromatography, and a developer with a volume ratio of V (ethyl acetate): V (petroleum ether) = 1:3 was selected. After the reaction is complete, stop the reaction, let the reaction system drop to room temperature slowly, add 30mL of 3°C cold water, let it stand still, a solid precipitates, filter with suction, wash the filter cake repeatedly until it is neutral, recrystallize it with absolute ethanol, and evaporate it with a rotary e...
Embodiment 3
[0052] Weigh 0.006mol cyclohexanone, 0.006mol NaOH (solid, granular), and 30mL of absolute ethanol into a dry three-necked flask equipped with a condensing reflux tube and a stirring device, and stir at room temperature for 5 minutes. After mixing evenly, add 0.001mol of 1-(3-N-methyl-carbazolyl)-3-(p-methylphenyl)-propenone, stir and slowly raise the temperature to 80°C, and make it Reflux reaction 75min under the temperature of 70rad / min stirring speed. During the reaction period, the reaction progress was monitored by thin-layer chromatography, and a developer with a volume ratio of V (ethyl acetate): V (petroleum ether) = 1:3 was selected. After the reaction is complete, stop the reaction, let the reaction system drop to room temperature slowly, add 30mL of 3°C cold water, let it stand still, a solid precipitates, filter with suction, wash the filter cake repeatedly until it is neutral, recrystallize it with absolute ethanol, and evaporate it with a rotary evaporator. Rem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com