Preparation method of imidazo (1, 5-alpha) pyridine derivative DH2PIP and complex thereof
An imidazo and derivative technology is applied in the field of preparation of imidazo[1,5-α]pyridine derivatives DH2PIP and its complexes, which can solve the problems of harsh reaction conditions and high reaction cost, and achieves low cost and preparation method. Simple, wide-ranging effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
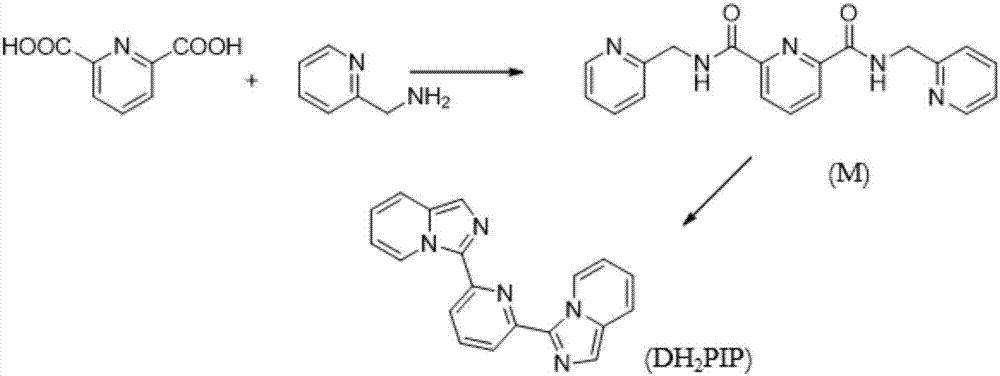
[0029] The invention provides imidazo[1,5-α]pyridine derivative DH 2 The preparation method of PIP, the method comprises the steps:
[0030] (1) Preparation of intermediate product M;
[0031] (2) Preparation of product DH 2 PIP.
[0032] In order to make the above objects, features and advantages of the present invention more comprehensible, the present invention will be further described in detail below in conjunction with specific embodiments.
[0033] Imidazo[1,5-α]pyridine derivative DH 2 The preparation method of PIP comprises:
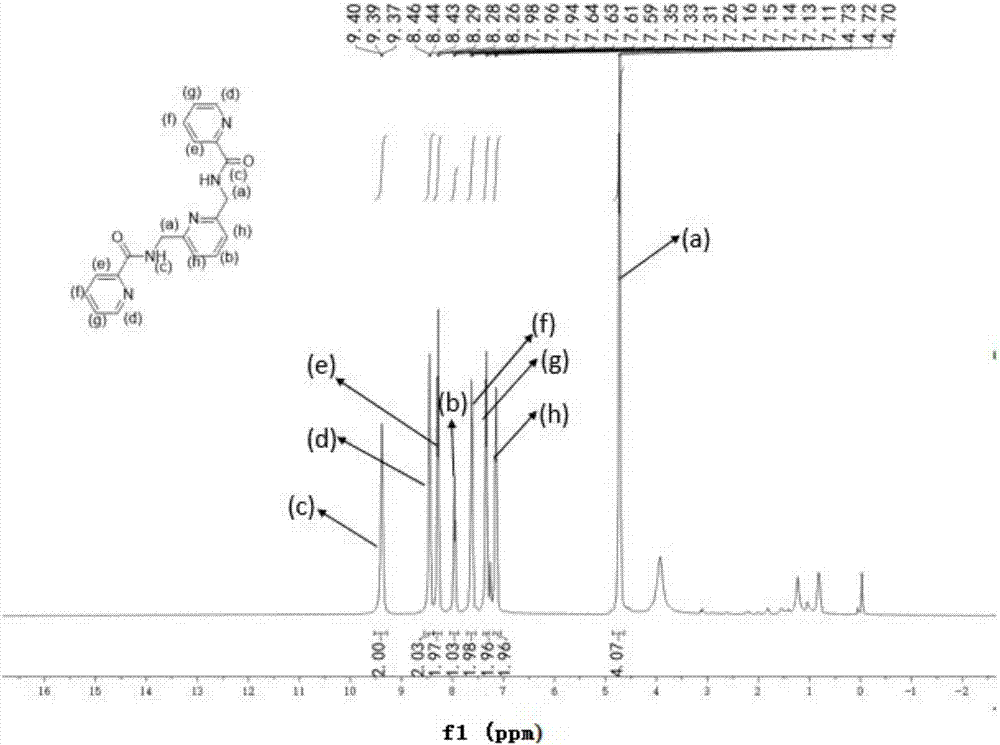
[0034] Step 1: Weigh 2,6-pyridinedicarboxylic acid into a flask, add ethyl n-butyrate and stir to dissolve, slowly add 2-aminomethylpyridine while stirring, stir at room temperature for half an hour, then add tripropylene base phosphoric anhydride, and then put it into a 130°C oil bath and heat it to reflux for six hours. After cooling to room temperature, the reacted liquid is divided into upper and lower layers, and the lower layer is ret...
Embodiment 1
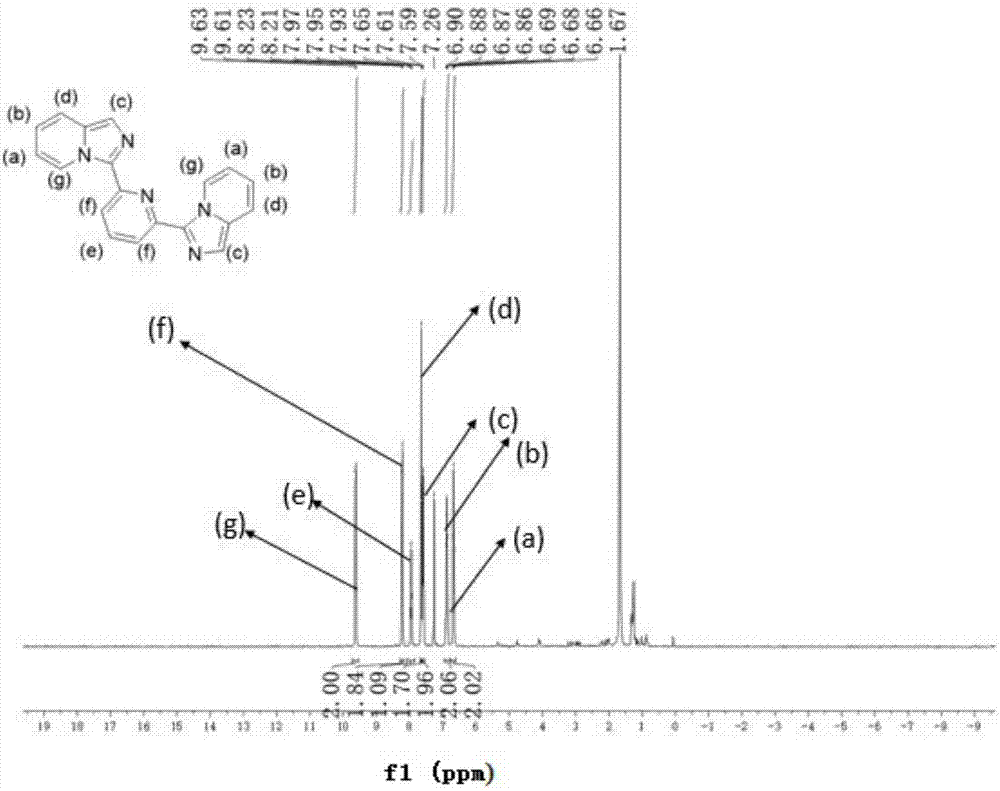
[0044] This implementation case shows the preparation method of imidazo[1,5-α]pyridine derivative DH2PIP (C19N5H13) according to the following steps:
[0045] Accurately weigh 2,6-pyridinedicarboxylic acid (20 mmol, 3.34 g) into a 250 mL round-bottomed flask, add 20 mL of ethyl n-butyrate and stir to dissolve, slowly add 2-aminomethylpyridine (40 mmol , 4.32 g), stirring while adding, after stirring at room temperature for half an hour, add 10 mL of tripropylphosphoric anhydride (T3P), put it in a 130 ℃ oil bath and heat to reflux for six hours, after cooling to room temperature, it can be It can be seen that the reacted liquid is divided into upper and lower layers, the upper layer is dark yellow clear liquid, and the lower layer is dark brown viscous. Post-treatment: use a straw to separate out the supernatant and discard it, keep the lower layer for use, add an appropriate amount of water to the lower layer, then adjust the pH with saturated sodium bicarbonate solution, sti...
Embodiment 2
[0049] This implementation case shows the preparation method of the complex [Cu(DH2PIP)Cl2] (C19H13N5Cl2Cu) of imidazo[1,5-α]pyridine derivative DH2PIP according to the following steps:
[0050] Accurately weigh the ligand DH2PIP (0.0397 g, 0.1 mmol) and copper chloride dihydrate (0.0171 g, 0.1 mmol), add them to the glass tube in turn, dissolve the mixture with 2 mL of absolute ethanol, put it in a constant temperature drying oven and heat up to 125 oC, under the action of autogenous pressure, keep the temperature at constant temperature for 2 days, turn off the oven, and let it cool down to room temperature naturally. Brownish yellow blocky crystals are formed. Filter, wash with absolute ethanol, and dry. Crystal yield: 70% (0.0310 g).
[0051] [Cu(DH2PIP)Cl2] (C19H13N5Cl2Cu) prepared in this example, the test results can be found in Figure 4 , Figure 4 It is the X-ray diffraction pattern of the complex [Cu(DH2PIP)Cl2] in the preparation method of the complex of imidazo[1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com