Specific-morphology Co3O4-loaded platinum catalyst and application thereof in low-carbon alcohol synthesis reaction through CO2 hydrogenation
A catalyst and morphology technology, applied in the direction of physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, reduction of oxygen-containing compounds, etc., can solve the problem that homogeneous catalysts are not easy to recycle and reuse, and pollute the environment and other problems, to achieve the effect of easy scale-up, high selectivity, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-2
[0031] Example 1-2: Preparation of nanorods or hexagonal nanodisks Co 3 o 4 Supported Pt catalyst
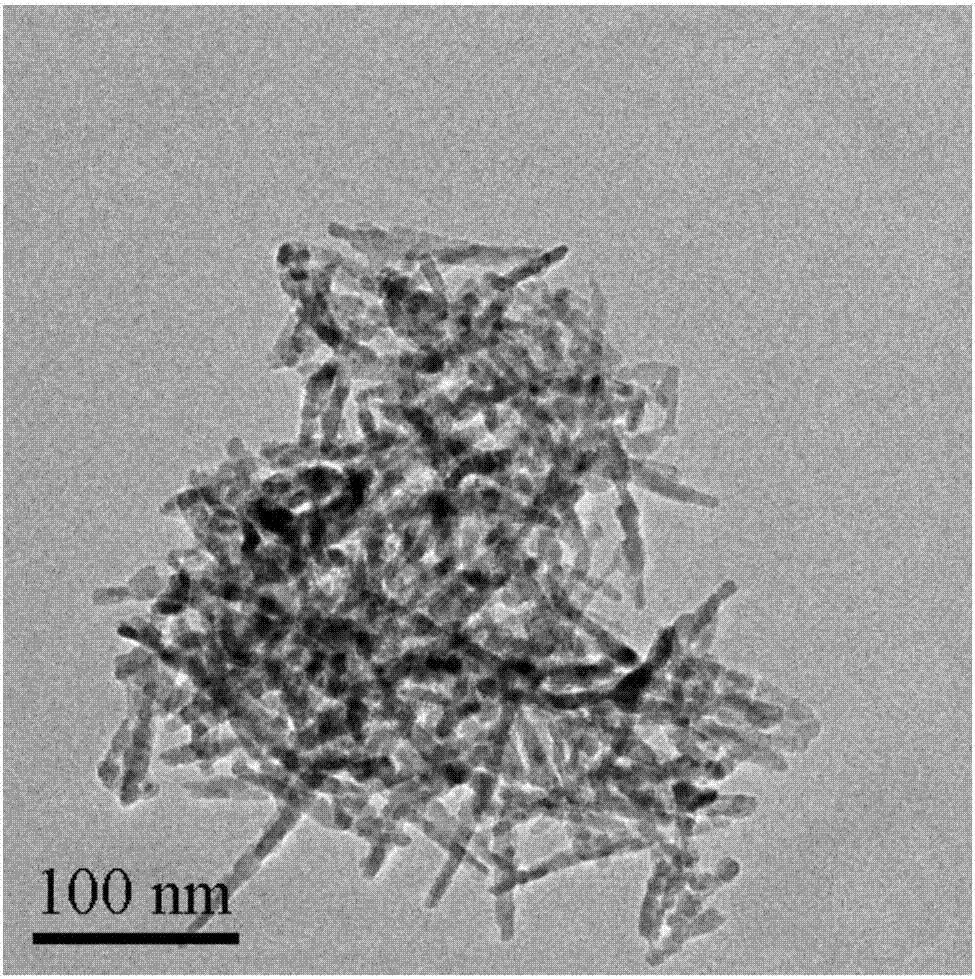
[0032] (1) 4.98g Co(Ac) 2 4H 2 O was dissolved in 60ml of ethylene glycol, heated to 160°C, and 0.2mol / L of Na was added dropwise 2 CO 3 solution, the consumption of 0.2mol / L Na 2 CO 3 The volume of the solution is 200ml. After the dropwise addition, the suspension is aged for 1h, filtered, and the obtained filter cake is washed three times with deionized water, and the obtained solid is dried in a vacuum oven at 50°C for 12h. ℃ in a muffle furnace for 4 h to obtain nanorods Co 3 o 4 , referred to as Co 3 o 4 -r.
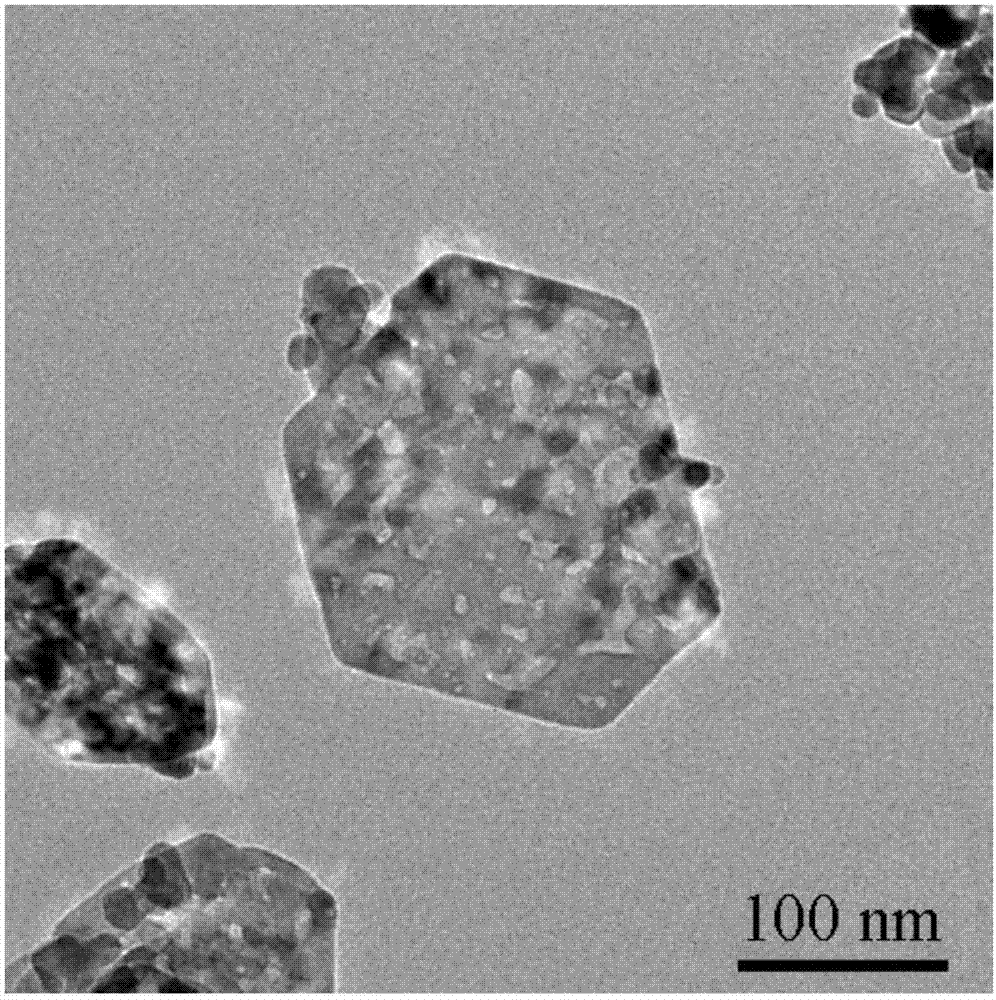
[0033] (2) 2mmol Co(NO 3 ) 2 ·6H 2 O was dissolved in 40ml of water to form a red solution, 4ml of oleylamine and 20ml of ethanol were added to the red solution, and magnetically stirred for 30min to obtain a green solution. Add the green solution to a crystallization tank at 180°C for crystallization for 12 hours, centrifuge, and wash the resulting sol...
Embodiment 3-4
[0037] Using Pt / Co 3 o 4 -r catalyzed CO 2 The method for hydrogenation synthesis of low-carbon alcohols, the steps are:
[0038] The Pt / Co prepared by 0.2g embodiment 1 3 o 4 The -r catalyst was mixed with 0.2g SiC evenly and added to a self-made fixed bed reactor (made of stainless steel, 47cm long, 1 / 4 inch inner diameter), at 200°C, H 2 After 2 hours of normal pressure reduction under the atmosphere, the reactor was cooled to room temperature, and the volume fraction was 22.5% CO 2 , 67.5%H 2 , 10%N 2 The mixed gas, the reactor pressure is increased to 2MPa within 3h, and the space velocity is 6Lg cat -1 h -1 , Then the reactor temperature was raised to 190° C. (Example 3) and 200° C. (Example 4) respectively, the heating rate was 2° C. / min, and the timing of the reaction time was started after reaching this temperature. The gas phase product was analyzed online by Agilent GC7890B, and the data after the reaction was stable for 50 hours was selected to calculate ...
Embodiment 5-6
[0045] Embodiment 5-6: same as embodiment 3-4, just change catalyst to be the nano-disk Co prepared by embodiment 2 3 o 4 Supported Pt catalyst (Pt / Co 3 o 4 -p), the test results are shown in Table 3 and 4. It can be seen that at the same reduction temperature and CO 2 Compared with nanorod-supported catalysts at the hydrogenation reaction temperature, the use of hexagonal nanodisks Co 3 o 4 When supporting Pt catalyst, CO 2 The conversion rate decreased slightly, the selectivity of C2 and above hydrocarbons decreased, and the selectivity of methane and alcohols increased. with Pt / Co 3 o 4 The results of -r are similar. When the temperature is increased from 190 °C to 200 °C, the selectivity of total alcohols decreases, but the proportion of low-carbon alcohols in total alcohols increases.
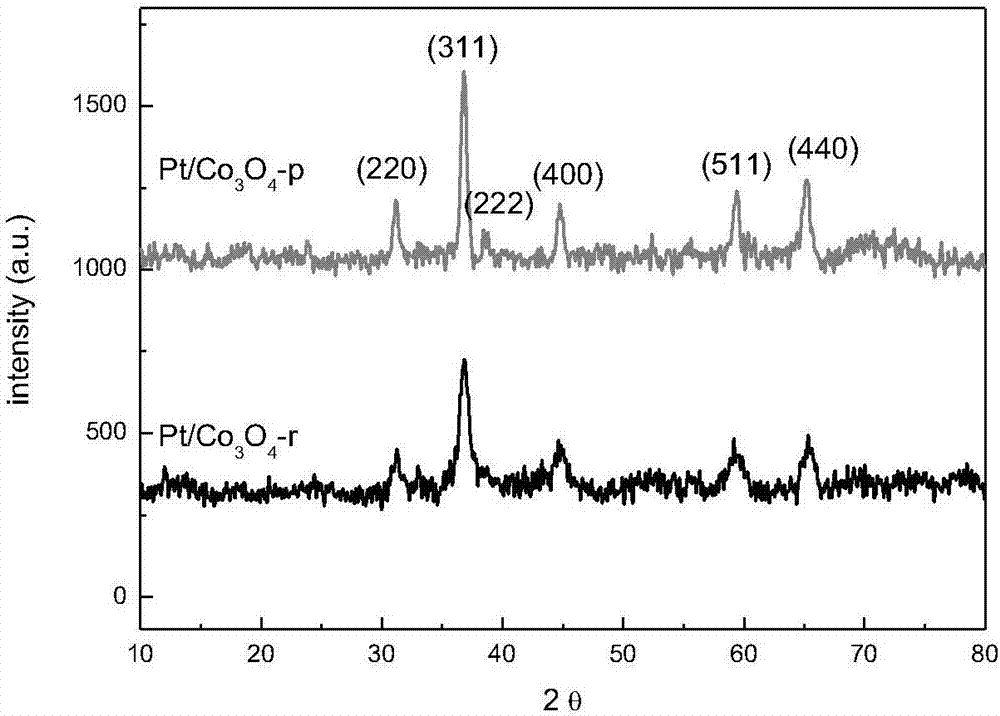
[0046] Figure 4 For Pt / Co 3 o 4 -r, Pt / Co 3 o 4 The XRD pattern of the -p catalyst after hydrogen reduction at 200°C, from Figure 4 It can be seen that the morphology of t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com