Method and device for preparing synthesis gas and capturing and utilizing carbon dioxide
A technology of carbon dioxide and synthesis gas, applied in hydrogen/synthesis gas production, chemical instruments and methods, petrochemical industry, etc., to achieve the effect of reducing energy consumption demand
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
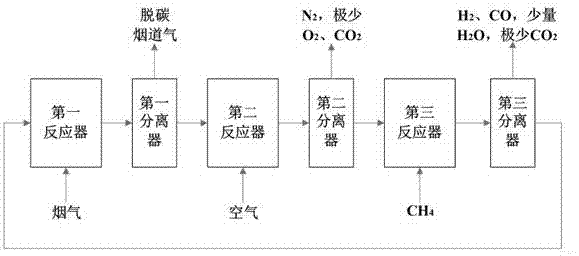
[0039] This example provides a synergistic capture and utilization of flue gas CO 2 The device and method, respectively with CaO and Fe as CO 2 Carrier and O 2 Carrier as an example, the specific process is as follows: To CO 2 A stream of dry flue gas from a 600MWe coal-fired power station is fed into the trap, the gas flow rate is 1 kmol / s, and the gas content is: 15% CO 2 , 5% O 2 and 80% N 2 . The solid component is the solid component after gas-solid separation from the fuel reactor, Fe feed is 0.80 kmol / s, CaO feed is 0.15 kmol / s, CO 2 The capture reactor temperature was 400 °C and the operating pressure was ~1 atm. The air feed to the oxidation reactor is 3.33 kmol / s, and the solid feed is the solid component separated by the first separator, in order to prevent CaCO 3 For decomposition, the oxidation reactor temperature is 600 °C and the operating pressure is ~1 atm. Reforming reactor CH 4 The feed rate is 1.70 kmol / s, the solid feed is the solid component sepa...
Embodiment 2
[0041] This example provides a synergistic capture and utilization of flue gas CO 2 The device and method, respectively with CaO and Ni as CO 2 Carrier and O 2 Carrier as an example, the specific process is as follows: To CO 2 A stream of dry flue gas from a 600MWe coal-fired power station is fed into the trap, the gas flow rate is 1 kmol / s, and the gas content is: 15% CO 2 , 5% O 2 and 80%N 2 . The solid component is the solid component after gas-solid separation from the reforming reactor, the feed rate of Ni is 0.20 kmol / s, the feed rate of CaO is 0.15 kmol / s, and the CO 2 The capture reactor temperature was 100 °C and the operating pressure was ~1 atm. The air feed to the oxidation reactor was 0.05 kmol / s O 2 and 0.19 kmol / s N 2 , the solid feed is the solid component separated by the first separator, in order to prevent CaCO 3 For decomposition, the oxidation reactor temperature is 600 °C and the operating pressure is ~1 atm. fuel reactor CH 4 The feed rate is ...
Embodiment 3
[0043] In this example, HSC 5.0 is used to study different oxygen carriers and different CO 2 The enthalpy change of the three reactors for co-reforming methane with the carrier was used to explore the total enthalpy change of the three reactors. CaCO 3 -CoO-CH 4 Combination as an example: the molar feed ratio is 1:1:1, in CO 2 The following reactions take place in the capture reactor:
[0044] CaO + CO 2 (g) = CaCO 3 ……(1)
[0045] The following reactions take place in the oxidation reactor:
[0046] Co + O 2 (g) = 1 / 2CoO...(2)
[0047] The following reactions take place in the reforming reactor:
[0048] CaCO 3 + CoO + CH 4 (g) = 2CO(g) + H 2 (g) + Co + CaO +H 2 O(g)...(3)
[0049] CO 2 Capture reactor operating temperature T 1 = 400°C, △H 1 = -174.391 kJ (where, T 1 、△H 1 The subscript of means the reaction in the first step, and applies to the second and third steps by analogy); in the oxidation reactor, in order to prevent CaCO 3 Decompose, and at t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com