A fluorescent probe for targeting and labeling eye lens epithelial cells, its preparation and application
A technology of fluorescent probes and epithelial cells, which is applied in the field of fluorescent sensing materials, can solve the problem that eye lens epithelial cells cannot be completely removed at one time, and achieve the effect of avoiding postoperative complications and inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
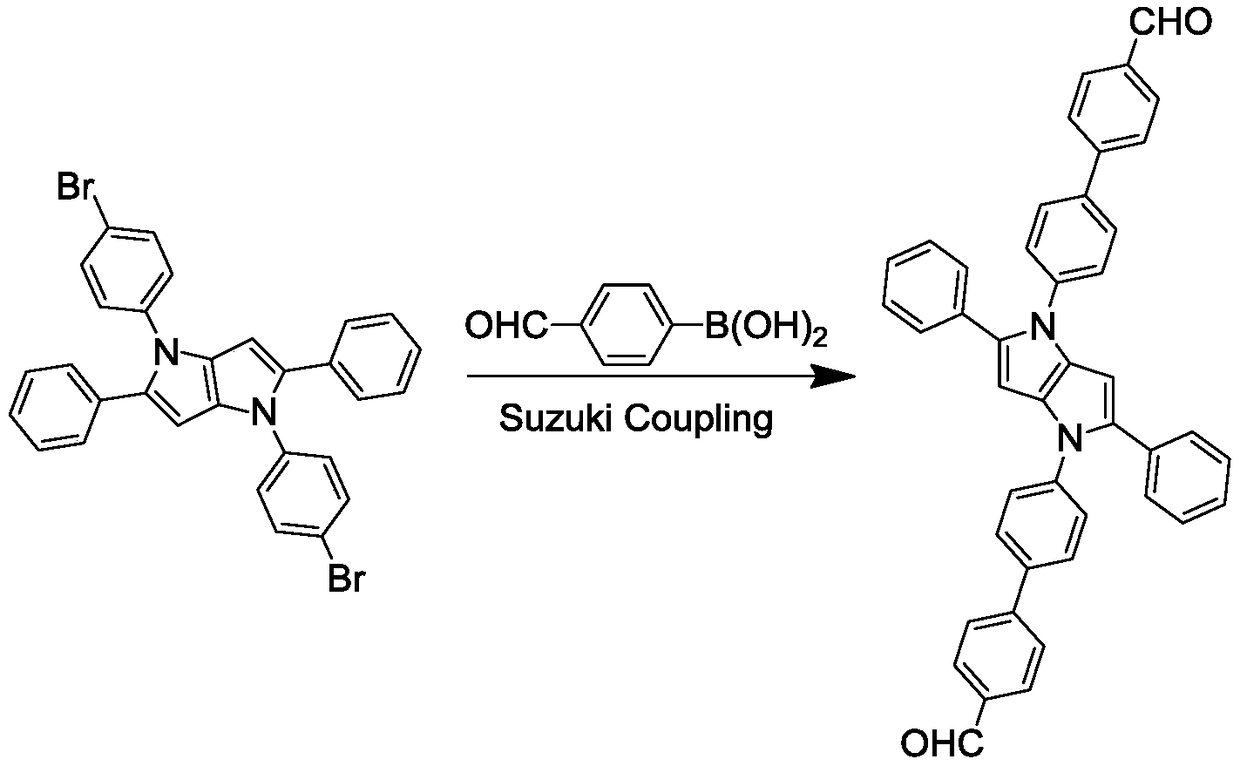
[0040] (1) Take 56.8mg (0.1mmol) of 1,4-bis(4-bromophenyl)-2,5-diphenyl-1,4-dihydropyrrolo[3,2-b]pyrrole, 45mg (0.3mmol) 4-formyl phenylboronic acid, 6mg (0.005mmol) tetrakis (triphenylphosphine) palladium, 67.5mg (0.5mmol) potassium carbonate were added to a 100mL two-necked round-bottomed flask, vacuumed and filled with nitrogen three times, and 12mL was added to remove A mixed solvent of toluene and methanol (volume ratio of toluene and methanol is 3:1), after reflux at 110°C for 8h, cool the reaction solution in the two-necked round-bottomed flask to room temperature, and then add a volume twice the volume of the reaction solution A mixture of water and dichloromethane (V 水 :V 二氯甲烷 =1:1) to extract the reaction solution, dry the separated organic phase with anhydrous magnesium sulfate, and then use a rotary evaporator to spin dry at 40°C to obtain a crude product; the crude product is separated and purified by column chromatography, and placed Dry it in a vacuum drying o...
Embodiment 2
[0051] (1) Take 113.6mg (0.2mmol) of 1,4-bis(4-bromophenyl)-2,5-diphenyl-1,4-dihydropyrrolo[3,2-b]pyrrole, 90mg (0.6mmol) 4-formyl phenylboronic acid, 12mg (0.01mmol) tetrakis (triphenylphosphine) palladium, 135mg (1mmol) potassium carbonate were added to a 100mL two-necked round-bottomed flask, evacuated and filled with nitrogen three times, and added 12mL of deoxygenated A mixed solvent of toluene and methanol (the volume ratio of toluene and methanol is 3:1), after reflux at 110°C for 8 hours, cool the reaction solution in the two-necked round bottom flask to room temperature, and then add water twice the volume of the reaction solution and dichloromethane mixture (V 水 :V 二氯甲烷 =1:1) to extract the reaction solution, dry the separated organic phase with anhydrous magnesium sulfate, and then use a rotary evaporator to spin dry at 40°C to obtain a crude product; the crude product is separated and purified by column chromatography, and placed Dry it in a vacuum oven at 25°C t...
Embodiment 3
[0064] (1) 0.95mg DPPHP-2CHO prepared in Example 1 was dissolved in 1.5mL DMSO to make a concentration of 1×10 -3 mol / L solution g, then take 0.1mL solution g and dilute it with DMEM to a concentration of 1×10-4 mol / L solution h, filter solution h with a microporous membrane with a pore size of 0.22 μm to obtain solution I;
[0065] (2) Passage the rabbit eye lens epithelial cells (BLE cells) and ICR mouse embryonic fibroblasts (MEF cells) that have covered the bottom of the culture dish to a small culture dish (Φ=20mm) with slides at the bottom. ), each group contains three parallel experiments, at 37℃, CO 2 Cultivate for 12 hours in an incubator with a volume fraction of 5%;
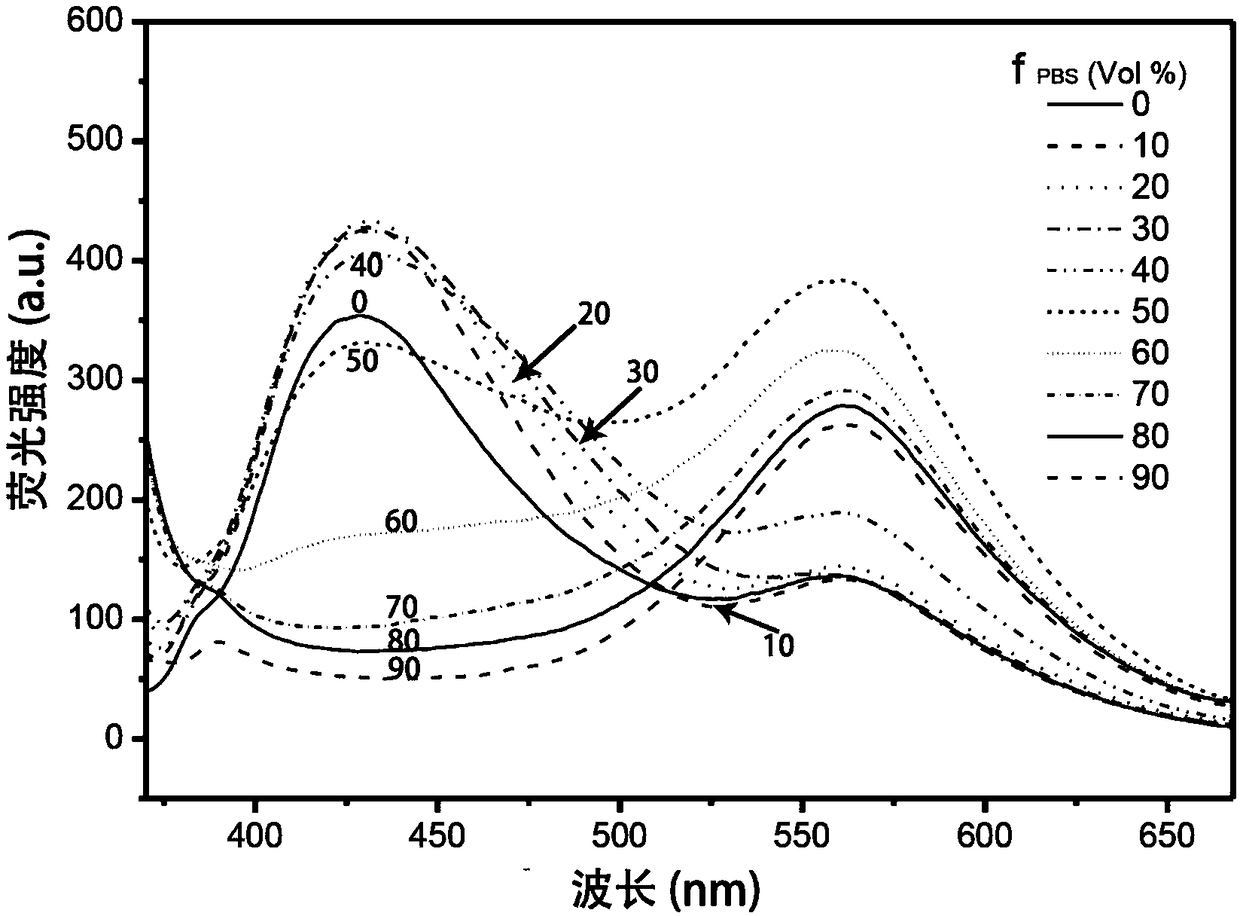
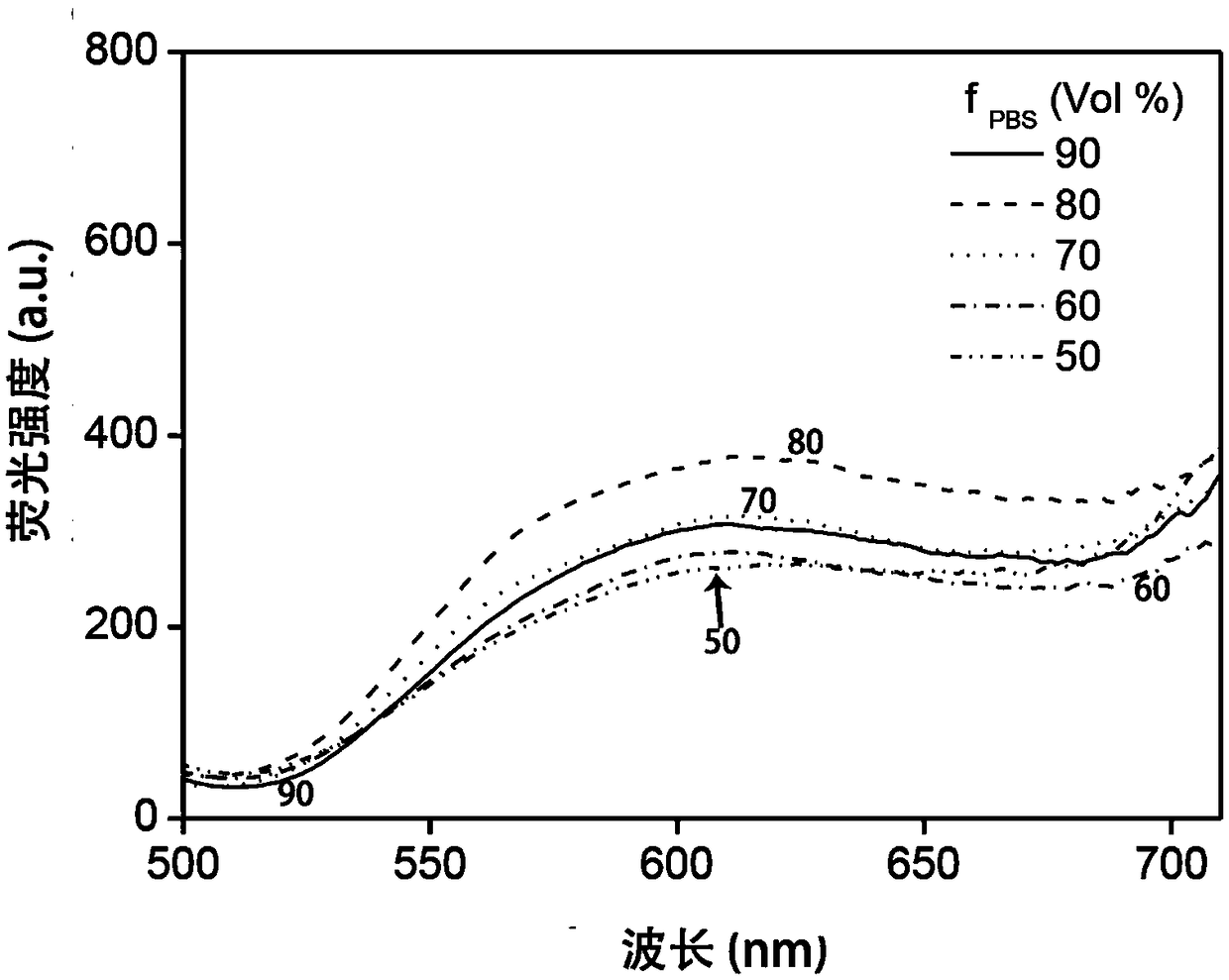
[0066] (3) Aspirate the medium from the culture dish containing the cells, add solution I with a volume of 0.5mL and place it under a laser confocal microscope (excitation wavelength 543nm, wavelength range 555-700nm) to observe the concentration of solution I in the BLE cell and MEF cell. Imaging si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com