Difunctional-group-modified chitosan derivative and preparation method thereof
A technology of chitosan derivatives and bifunctional groups, which is applied in the field of chitosan preparation, can solve the problems of reducing biological safety, affecting applications, and no obvious improvement in antibacterial properties, so as to avoid antibacterial function or biological safety Decrease, increase rapid penetration, improve the effect of water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
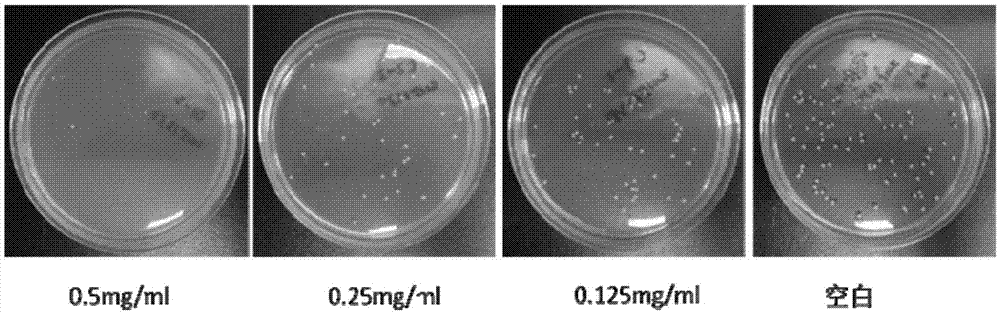
Examples
Embodiment 1
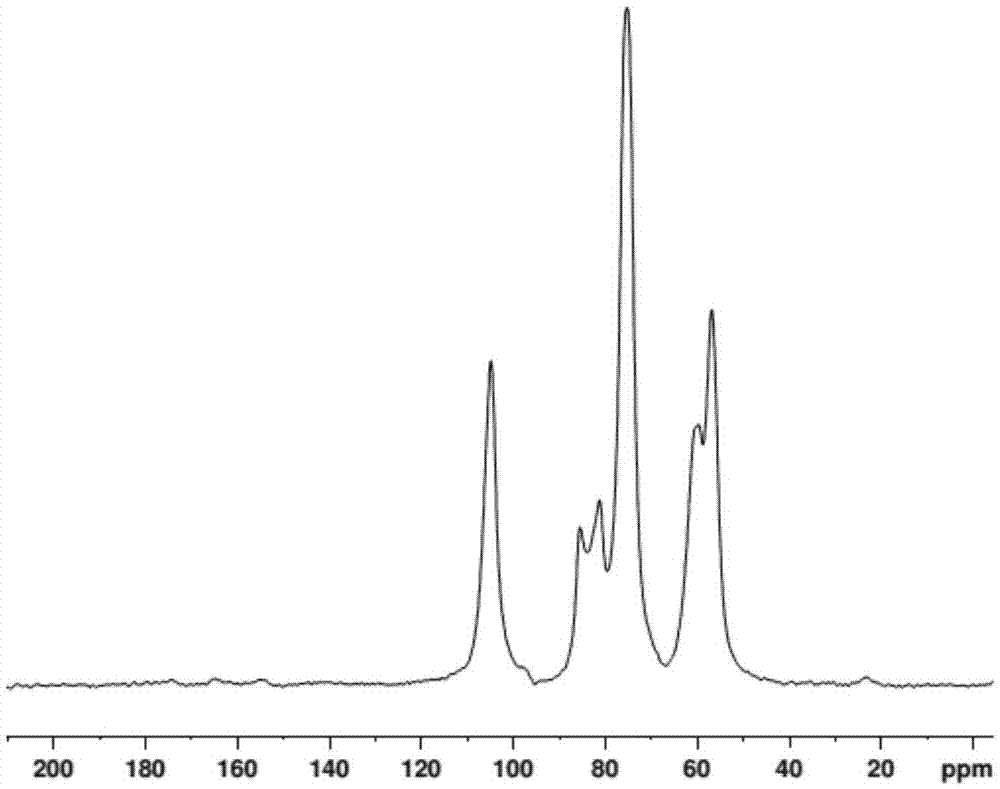
[0048] A bifunctional group modified chitosan derivative, its molecular structure is shown below
[0049]
[0050] Among them, x, y, n are natural numbers, 07 , 07 , 10 2 ≦n≦10 7 .
[0051] The specific preparation method of the above chitosan derivative is as follows:
[0052] Weigh 0.1 g of chitosan and add it to 100 ml of deionized water, and stir it mechanically for half an hour at room temperature to dissolve the chitosan completely, thereby obtaining a uniform solution with a concentration of 0.1% by mass and volume; Slowly add thiourea trioxide to the system, the molar ratio of thiourea trioxide to chitosan is 10:1, the feeding time is 30 minutes, and the reaction is maintained at room temperature for 60 minutes; then the refined is activated in an ice-water mixed bath for 3 hours A mixed solution of acid, N-hydroxysuccinimide (NHS) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) (solvent 30mmol / 20ml of 2-(N-morpholino)ethanesulfonic acid (MES) buf...
Embodiment 2
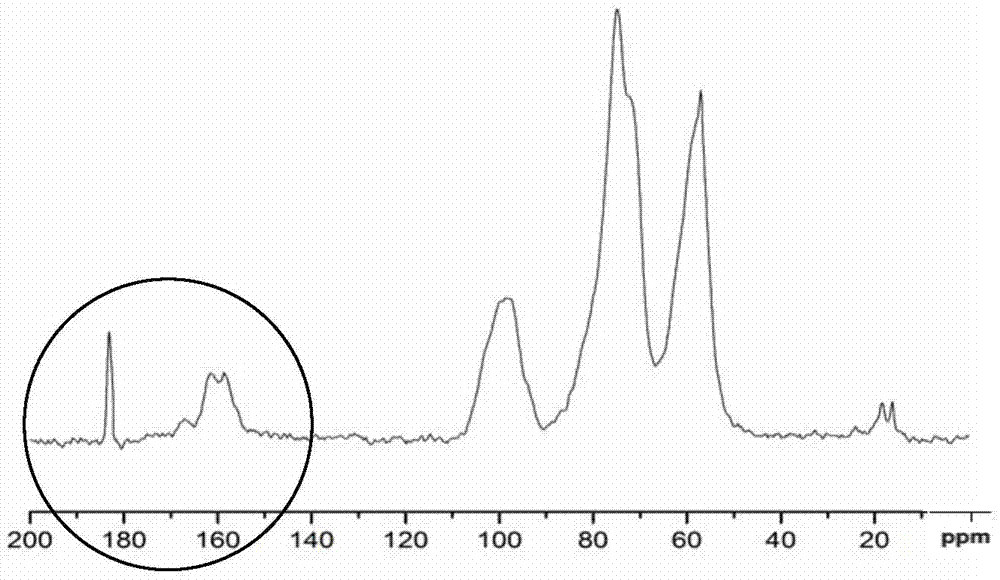
[0061] A bifunctional group modified chitosan derivative, its molecular structure is shown below
[0062]
[0063] Among them, x, y, n are natural numbers, 07 , 07 , 10 2 ≦n≦10 7 .
[0064] The specific preparation method of the above chitosan derivative is as follows:
[0065] Weigh 0.5 g of chitosan and add it to 100 ml of dilute hydrochloric acid with a concentration of 0.1 mol / L, and mechanically stir for half an hour at room temperature to dissolve the chitosan completely, thereby obtaining a uniform solution with a concentration of 0.5% by mass and volume; Next, slowly add thiourea trioxide to the dilute acid solution system of chitosan, the molar ratio of thiourea trioxide to chitosan is 5:1, the feeding time is 30 minutes, and the reaction is maintained at room temperature for 60 minutes; Arginine, N-hydroxysuccinimide (NHS) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) activated for 3 hours in a mixed bath 20ml of mixed solution (the solvent is ...
Embodiment 3
[0067] A bifunctional group modified chitosan derivative, its molecular structure is shown below
[0068]
[0069] Among them, x, y, n are natural numbers, 07 , 07 , 10 2 ≦n≦10 7 .
[0070] The specific preparation method of the above chitosan derivative is as follows:
[0071] Weigh 1.0 g of chitosan and add it to 100 ml of dilute hydrochloric acid with a concentration of 0.1 mol / L, and stir it mechanically for one hour at 35°C in a water bath to dissolve the chitosan completely, thereby obtaining a concentration of 1% by mass and volume. Homogeneous solution; slowly add thiourea trioxide to the dilute acid solution system of chitosan in a 35℃ water bath. The molar ratio of thiourea trioxide to chitosan is 4:1. The feeding time is 40 minutes, and the reaction is maintained at room temperature for 50 minutes. ; Then arginine, N-hydroxysuccinimide (NHS) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) activated at room temperature for 2 hours ·HCl) mixed solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com