A preparation and purification method of dehydrophenylahistine compounds
A technology of phenylahistine and purification method, applied in the direction of organic chemistry, etc., can solve the problems of complex preparation and purification methods and restrictions on the industrial production of drugs, and achieve the effects of good reproducibility, increased yield, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of crude (3Z,6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazol-4-yl)deuteromethylene)piperazine-2,5-dione
[0037] Its specific preparation process comprises the following steps:
[0038] 1) Preparation of (Z)-1-acetyl-3-((5-(tert-butyl)-1H-imidazol-4-yl)deuterated methylene)piperazine-2,5-dione
[0039] Add 10.00g (65.29mmol) 5-(tert-butyl)-1H-imidazole-4-deuteroformaldehyde to 50mL DMF, then add 25.88g (130.59mmol) N, N-diacetylpiperazine-2,5- The diketone was exhausted three times under nitrogen protection, 31.91g (97.94mmol) cesium carbonate was added, nitrogen protection was exhausted three times, and the reaction was stirred at room temperature for 20h in the dark. Pour the reaction solution into ice water (400mL), filter with suction, wash the filter cake with water (200mL*2), petroleum ether: ethyl acetate = 8:1 (200mL) successively, and ultrasonically disperse the filter cake with ethanol and dichloromethane , filtered off the insoluble matter, conce...
Embodiment 2
[0043] (3Z,6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazol-4-yl)deuteromethylene)piperazine-2,5-dione monohydrate preparation
[0044]
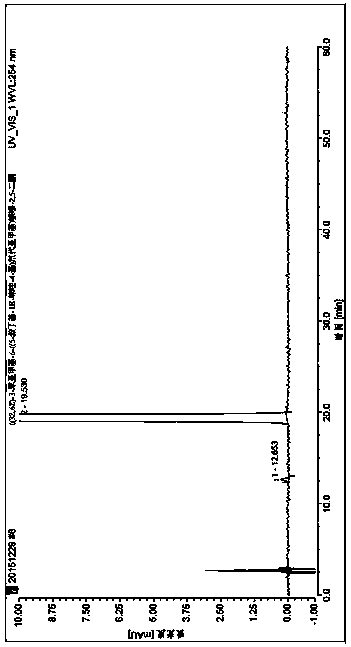
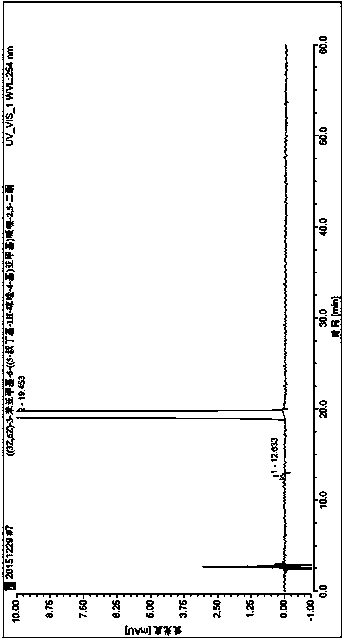
[0045] Put 2.0 g of the crude product described in Example 1 in a brown bottle, add 125 mL of isopropanol under heating conditions until completely dissolved, then add 50 mL of water without crystallization, place at room temperature, stir, cool and crystallize, and filter with suction. Virahol: water=1: 1 washes filter cake, dries, obtains yellow powdery solid 1.642g, and yield is 78.13%, and the purity of product under 254nm is 99.94%, wherein isomer ((3E, 6Z)- 3-benzylidene-6-((5-tert-butyl-1H-imidazol-4-yl)deuteromethylene)piperazine-2,5-dione 0.06%, see figure 1 . 1 H NMR (500MHz, dmso) δ 12.22 (brs, 2H), 10.00 (brs, 1H), 7.82 (d, J = 12.7Hz, 1H), 7.51 (d, J = 7.6Hz, 2H), 7.40 (t , J=7.7Hz, 2H), 7.30(t, J=7.4Hz, 1H), 6.73(s, 1H), 1.37(s, 9H). MS(ESI)m / z 338.1715(M+H) + (calcd for C 19 h 20 DN 4 o 2 ).
[0046] The resulting p...
Embodiment 3
[0050] (3Z, 6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazol-4-yl)methylene)piperazine-2,5-dione crude product preparation method
[0051] Its specific preparation process technology includes the following steps:
[0052] 1) Preparation of ethyl 5-(tert-butyl)oxazole-4-carboxylate
[0053] Add 90g (796mmol) ethyl isocyanoacetate to 1000mL tetrahydrofuran, slowly dropwise add 145g (955mmol) DBU, then dropwise add 178g (955mmol) trimethylacetic anhydride, and stir the reaction at room temperature for 48h after dropping. After the reaction was completed, it was concentrated under reduced pressure. For extraction, add an appropriate amount of 1500mL of dichloromethane, wash with 800mL of 10% sodium carbonate, 800mL of 10% citric acid, and 800mL of saturated brine, and back-extract the aqueous phase twice with 1000mL of dichloromethane. The organic phases were combined, dried over anhydrous sodium sulfate, filtered with suction after half an hour, and concentrated under reduced pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com