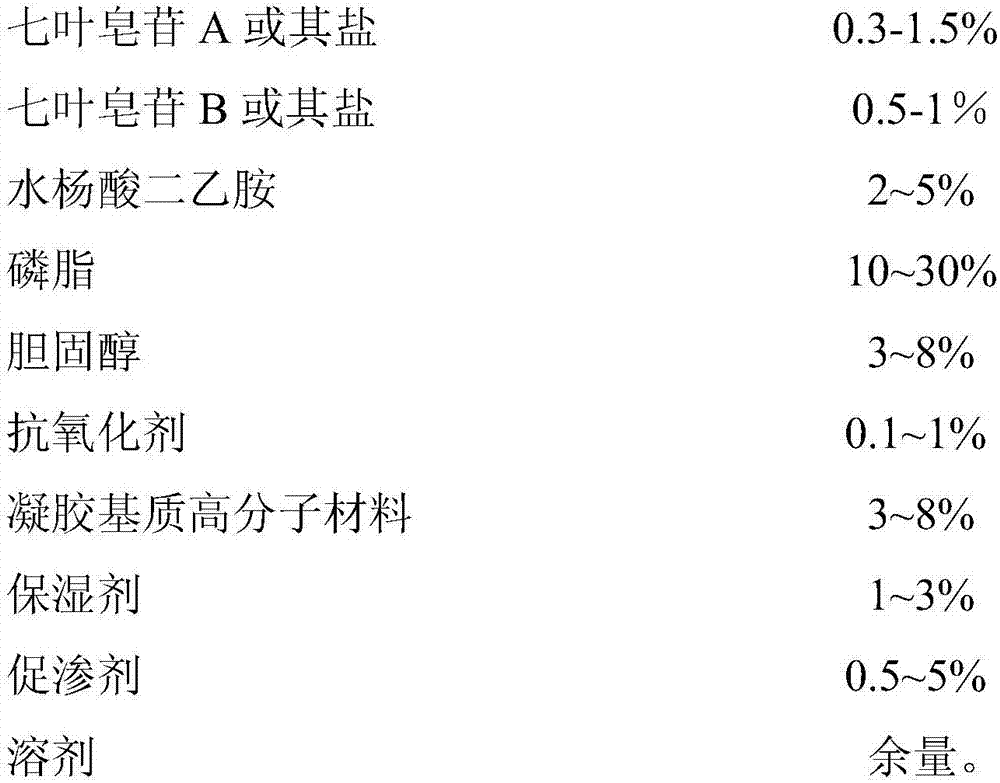
Compound aescine A and B liposome hydrogel patch
A compound aescin and hydrogel patch technology, which is applied in liposome delivery, anti-inflammatory agents, pharmaceutical formulations, etc., can solve the problem of unsatisfactory anti-swelling effect, poor transdermal absorption effect, large skin irritation, etc. problem, to achieve good transdermal absorption effect, high total transmittance, and low skin irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] Preparation method: 1) Take 25g of soybean lecithin, 5g of cholesterol, and 0.5g of ascorbic acid in a container, add 200g of 30% ethanol solution to dissolve, then add 1g of aescin Aescin, 0.6g of aescin B, and 2.5g of diethylamine salicylate , mixed evenly, placed in a constant temperature water bath at a temperature of 45°C, and the solvent was removed by rotary evaporation until a thin film was formed, then PBS buffer solution with a pH of 6 was added, stirred and shaken until a suspension was formed, filtered, and the filtrate was collected to obtain a lipid Body solution 65g;
[0031] 2) Get 5g of Carbomer, add 60g of 30% ethanol solution and stir to fully swell, then add 2g of glycerin, 3.5g of azone and 65g of the liposome solution obtained in step 1), adjust the pH value to 6 with hydrochloric acid, continue to stir and mix Evenly and ultrasonically degassed to no bubbles, 135g of compound aescin liposome hydrogel was obtained, coated, dried until t...
Embodiment 2
[0033]
[0034]
[0035] Preparation method: 1) Take 15g of lecithin, 8g of cholesterol, and 0.2g of ascorbic acid in a container, add 150g of 40% ethanol solution to dissolve, add 1.2g of aescin A sodium salt, 0.5g of aescin B sodium salt, and salicylic acid Diethylamine 2g, mix well, place in a constant temperature water bath at 40°C, and remove the solvent by rotary evaporation until a thin film is formed, then add PBS buffer solution with a pH of 5.5, stir and shake until a suspension is formed, filter, and collect the filtrate , to obtain liposome solution 55g;
[0036] 2) Get 3 g of hydroxypropyl methylcellulose, add 70 g of 40% ethanol solution and stir to swell fully, then add 1.5 g of glycerin, 0.5 g of propylene glycol, 1.5 g of borneol, 1 g of peppermint and 55 g of the liposome solution obtained in step 1), Use hydrochloric acid to adjust the pH value to 5, continue to stir and mix, and ultrasonically degas until there are no bubbles to obtain 132g of compoun...
Embodiment 3
[0038]
[0039]
[0040] Preparation method: 1) Take 20g of lecithin, 3g of cholesterol and 0.8g of ascorbic acid in a container, add 150g of 25% ethanol solution to dissolve, then add 0.5g of aescin A lysine salt and 1g of aescin B lysine salt 1. Diethylamine salicylate 4g, mix evenly, place in a constant temperature water bath at 50°C, and remove the solvent by rotary evaporation to form a thin film, then add PBS buffer solution with a pH of 7, stir and shake until a suspension is formed, Filter, collect filtrate, obtain liposome solution 70g;
[0041] 2) Take 4g of sodium alginate and 2g of gelatin, add 60g of 25% ethanol solution and stir to swell fully, then add 1g of glycerin, 2g of azone and 70g of the liposome solution obtained in step 1), adjust the pH value to 5.5 with hydrochloric acid, continue Stir and mix well and ultrasonically degas until there are no bubbles to obtain 139g of compound aescin liposome hydrogel, coat it, dry it until the surface is solidif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com