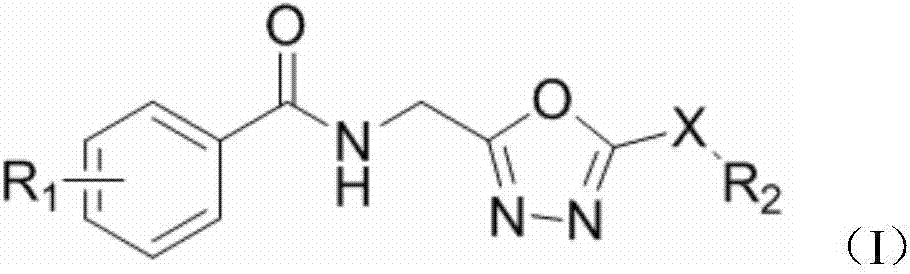
1,3,4-oxadiazole sulfur/oxygen ether compound with amide bonds, preparation method of compound and application
A technology of oxadiazole sulfur compounds, which is applied in the field of 1,3,4-oxadiazole sulfur/oxyether compounds and their preparation, can solve the problems of difficult prevention and control, wide range of damage, and low antiviral efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 of the present invention: Preparation of target compound 2-nitro-N-(2-methyl-5-(mercapto)-1,3,4-oxadiazole)benzamide
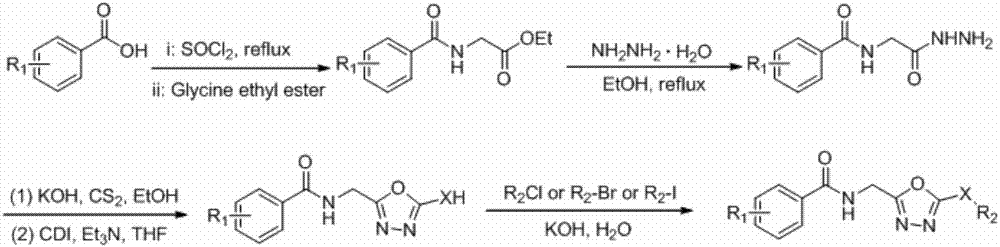
[0022] Add N-(2-acetylhydrazino)-2-nitrobenzamide (5.0mmol) into 50mL ethanol solution dissolved in KOH (10mmol), slowly add carbon disulfide (10.0mmol) dropwise, and react at room temperature for 12 hours Change to heating and reflux for 12 hours, stop the reaction, remove the solvent, add 30 mL of water, and adjust the pH to 3-4 with concentrated hydrochloric acid to obtain a yellow solid with a yield of 54.2% and a melting point of >230°C.
Embodiment 2
[0023] Example 2 of the present invention: Preparation of target compound 2-nitro-N-(2-methyl-5-(methylsulfide)-1,3,4-oxadiazole)benzamide
[0024] Add 2-nitro-N-(2-methyl-5-(mercapto)-1,3,4-oxadiazole)benzamide (2.0mmol) into NaOH solution, add dimethyl sulfate dropwise The ester (2.0 mmol) was reacted at room temperature for 12 hours, then the reaction was stopped, and the reaction was suction filtered to obtain a yellow solid with a yield of 31.4% and a melting point of 108-109°C.
[0025] The synthesis of other target compounds refers to Example 1 or 2.
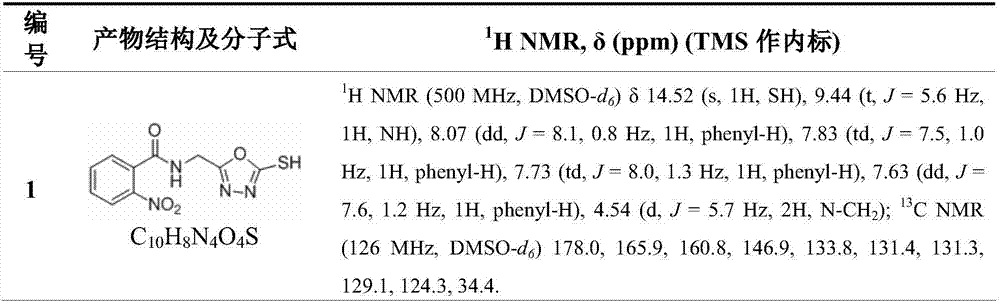
[0026] The structure, H NMR spectrum and carbon spectrum data of the synthesized 1,3,4-oxadiazole sulfur / oxygen ether compounds containing some amide bonds are shown in Table 1, and their physical and chemical properties are shown in Table 2.
[0027] H NMR spectrum and carbon spectrum data of some compounds in Table 1
[0028]
[0029]
[0030] Table 2 Physicochemical properties of some target compounds
[0031] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com