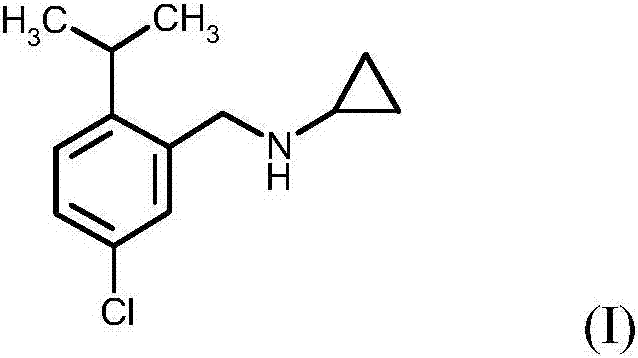
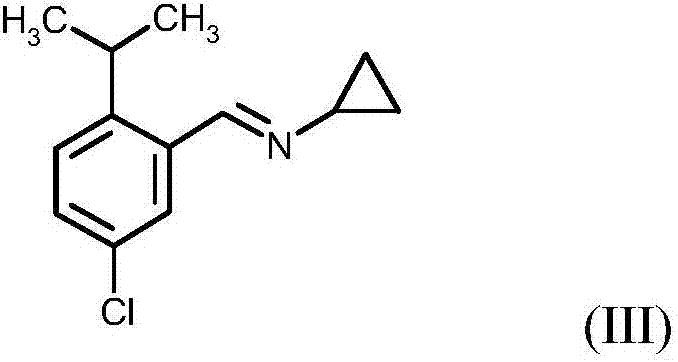
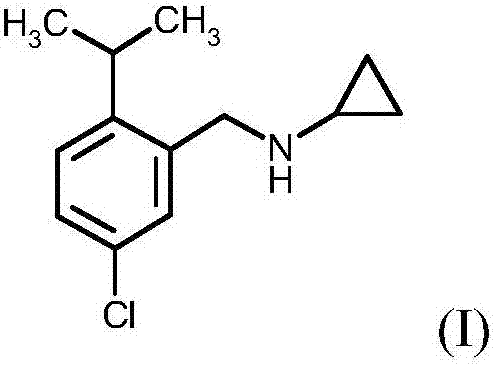
Process for preparing N-(5-chloro-2-isopropylbenzyl)cyclopropanamine
A technology of isopropyl and cumene, applied in the field of preparing N-cyclopropylamine, can solve the problem of high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0206] Embodiment 1: 2-bromo-1-isopropyl-4-nitrobenzene of general formula (XI)
[0207] 1,3-Dibromo-5,5-dimethylhydantoin (86.9 g, 0.298 mol) was added to p-nitrocumene (X) (100 g , 0.581mol, GC purity 96%) and sulfuric acid (178g, 1.743mol, 98% strength) solution. The mixture was then warmed to room temperature and stirred for an additional 1 hour. The reaction mixture was poured onto 200 g of ice water and mixed with sodium disulfide (15.1 g, 0.06 mol) and toluene (300 g). The phases are then separated. The organic phase was washed with 5% strength aqueous sodium hydroxide solution and the toluene was distilled off under reduced pressure. 2-Bromo-1-isopropyl-4-nitrobenzene was obtained as a pale yellow oil (147 g, 93.6 GC-%, based on area, 97% of theory).
[0208] A solution of p-nitrocumene (X) (50 g, 0.300 mol, GC purity: 99.1%) and iron (III) chloride was heated to 40° C. and bromine (59.92 g, 0.375 mol) was added dropwise within 3 hours. The reaction was poured i...
Embodiment 2
[0211] Embodiment 2: 3-bromo-4-isopropylaniline of general formula (VII)
[0212] A solution of sodium sulfide (31.2 g, 0.24 mol) and sulfur (7.7 g, 0.24 mol) was stirred at 80° C. for 15 minutes. Isopropanol (160 g) was then heated and the mixture was stirred at 75° C. for another 15 minutes, and finally 2-bromo-1-isopropyl-4-nitrobenzene (XI) (50 g, 0.19 mol ). After stirring for an additional 5 hours, the reaction was complete. For work-up, the isopropanol was first distilled off and the remaining mixture was extracted with toluene / chlorobenzene. The combined organic phases were distilled off under reduced pressure. 3-Bromo-4-isopropylaniline was obtained as a red oil (42 g, 91 GC-%, based on area, 93% of theory).
[0213] 2-Bromo-1-isopropyl-4-nitrobenzene (XI) (20 g, 79.1 mmol, 96.5 GC%, based on area), methanol (400 ml), platinum on carbon (1% platinum, 2 % of alum, wet with water) (1.0g, 0.018 mmol) and zinc dibromide (90 mg, 0.40 mmol) were placed in a 600ml aut...
Embodiment 3
[0221] Embodiment 3: 2-bromo-4-chloro-1-cumene of general formula (IV)
[0222] Hydrochloric acid (152 g, 31% strength, 1.29 mol) was added dropwise to a suspension of 3-bromo-4-isopropylaniline (VII) (100 g, 0.43 mol) and water (150 g) at room temperature. The suspension was then cooled to 5°C and a solution of sodium nitrite (32.7 g, 0.46 mol) in water (140 g) was added dropwise within 2 hours. After stirring for a further 1 hour, sulfamic acid (2.5 g, 0.026 mol) was added. Copper (I) chloride (10.8 g, 0.11 mol), hydrochloric acid (202 g, 31%, 1.72 mol) and water (75 g) were added to a second flask, and the previously formed diazonium salt was added dropwise over 30 minutes . After stirring for a further 1.5 hours, the mixture was extracted with dichloromethane (250 g), the phases were separated and the dichloromethane was distilled off under reduced pressure. The obtained crude product was purified by distillation. 2-Bromo-4-chloro-1-isopropylbenzene was obtained as a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com