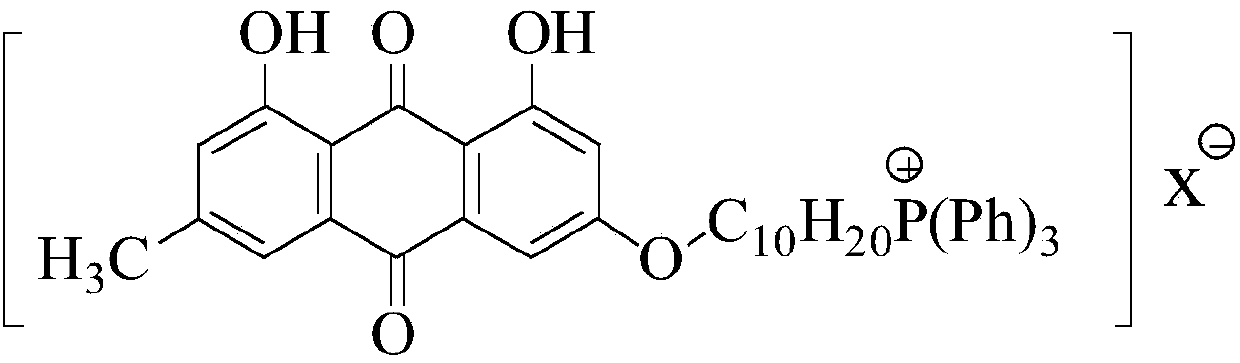
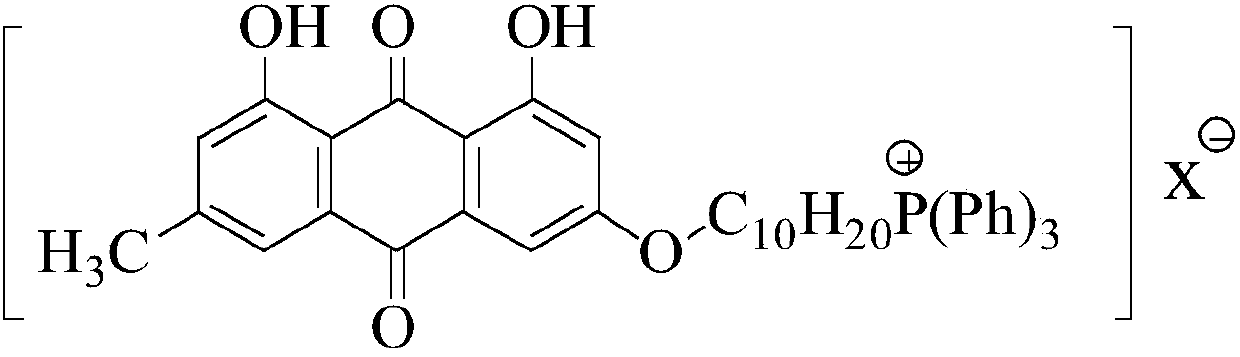
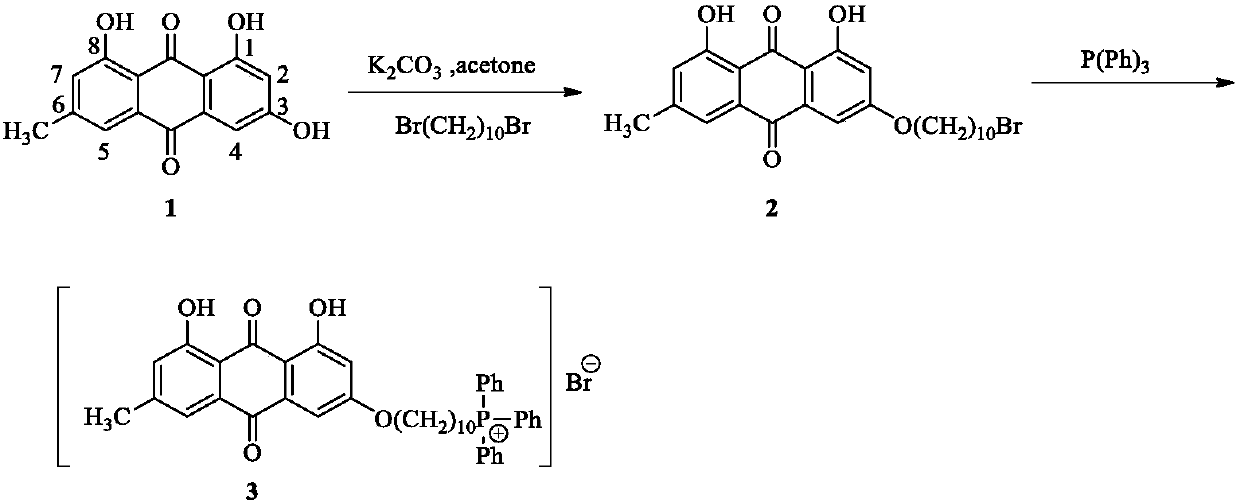
Emodin quaternary phosphonium salt derivative with antitumor activity, synthesis method therefor and application of emodin quaternary phosphonium salt derivative
A technology for antitumor activity and synthesis method, which is applied in the field of emodin quaternary phosphonium salt derivatives and synthesis thereof, and achieves the effects of mild conditions, simple synthesis method and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of intermediate 2 (3-bromodecyloxyemodin)——(a)
[0027] Take 1.6g (5.8mmol) of emodin 1 and dissolve it in 120ml of acetone, add 800mg (5.8mmol) of anhydrous potassium carbonate, heat to reflux, and slowly add 1.30ml (5.8mmol) of 1,10 -Dibromodecane, reflux for 5h. Cool to room temperature, remove acetone by rotary evaporation, add 100ml of water and stir for 25min, filter with suction to obtain a yellow powder crude product, the yellow powder crude product is in the condition of dichloromethane:petroleum ether (v / v)=1:2 as eluent Then, silica gel column chromatography was carried out to obtain a bright yellow solid, that is, 926 mg of 3-bromodecyloxyemodin, with a yield of 32.7%. The characterization data of intermediate product 2 (3-bromodecyloxyemodin) are as follows:
[0028] 1H NMR (400MHz, CDCl3): 12.35(s, 1H, Ar-OH), 12.17(s, 1H, Ar-OH), 7.64(d, J=1.6Hz, 1H, Ar-H), 7.38(d, J=2.4Hz, 1H, Ar-H), 7.11(d, J=1.6Hz, 1H, Ar-H), 6.69(d, J=2.4Hz, 1H, Ar-H), 4....
Embodiment 2
[0030] Synthesis of intermediate product 2 (3-bromodecyloxyemodin)—(b)
[0031] Take 3.2g (11.6mmol) of emodin 1 and dissolve it in 240ml of ethylene glycol monomethyl ether, add 1600mg (11.6mmol) of anhydrous potassium carbonate, heat to reflux, and slowly add 2.60ml (11.6mmol) of ) of 1,10-dibromodecane, refluxing for 3.5h. Cool to room temperature, remove ethylene glycol monomethyl ether by rotary evaporation, add 200ml of water and stir for 35 minutes, filter with suction to obtain a yellow powder crude product, which is prepared in dichloromethane:petroleum ether (v / v)=1:1.5 Under the condition of eluent, silica gel column chromatography was carried out to obtain a bright yellow solid, that is, 1860 mg of 3-bromodecyloxyemodin, with a yield of 32.8%. The characterization data of intermediate product 2 (3-bromodecyloxyemodin) are as follows:
[0032] 1H NMR (400MHz, CDCl3): 12.35(s, 1H, Ar-OH), 12.17(s, 1H, Ar-OH), 7.64(d, J=1.6Hz, 1H, Ar-H), 7.38(d, J=2.4Hz, 1H, Ar-H),...
Embodiment 3
[0034] Synthesis of intermediate product 2 (3-bromodecyloxyemodin)——(c)
[0035]Take 2.4g (8.7mmol) of emodin 1 and dissolve it in 180ml of acetone, add 1200mg (8.7mmol) of anhydrous potassium carbonate, heat to reflux, and slowly add 1.95ml (8.7mmol) of 1,10 -Dibromodecane, reflux 4h. Cool to room temperature, remove acetone by rotary evaporation, add 150ml of water and stir for 30min, filter with suction to obtain a yellow powder crude product, the yellow powder crude product is in the condition of dichloromethane:petroleum ether (v / v)=1:1 as eluent Next, silica gel column chromatography was carried out to obtain a bright yellow solid, namely 1400 mg of 3-bromodecyloxyemodin, with a yield of 32.9%. The characterization data of intermediate product 2 (3-bromodecyloxyemodin) are as follows:
[0036] 1H NMR (400MHz, CDCl3): 12.35(s, 1H, Ar-OH), 12.17(s, 1H, Ar-OH), 7.64(d, J=1.6Hz, 1H, Ar-H), 7.38(d, J=2.4Hz, 1H, Ar-H), 7.11(d, J=1.6Hz, 1H, Ar-H), 6.69(d, J=2.4Hz, 1H, Ar-H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com