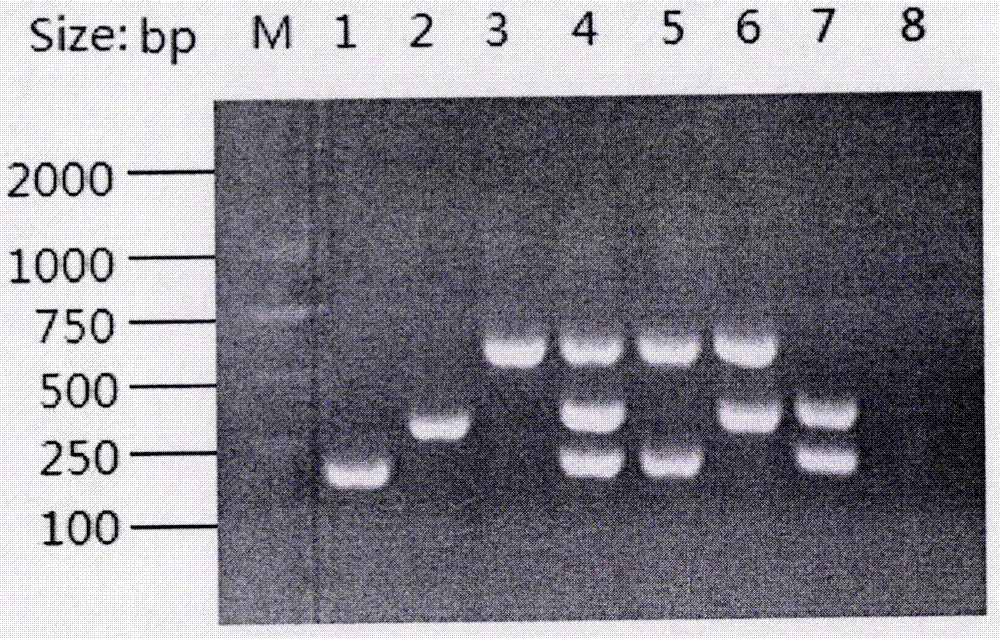
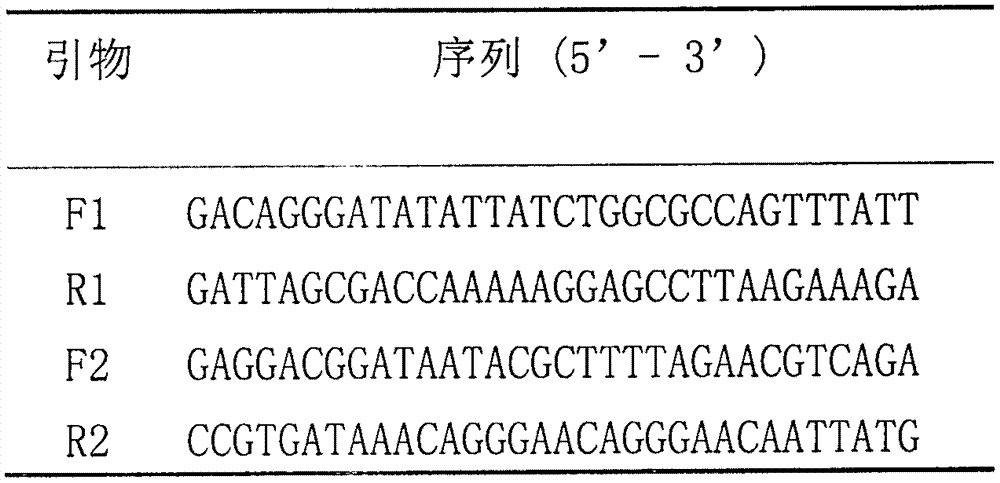
Primers for RPA rapid detection of Shigella and tetracycline drug resistance genes
A drug-resistant gene, Shigella technology, applied in the determination/inspection of microorganisms, DNA/RNA fragments, recombinant DNA technology, etc., can solve the problems of low accuracy, long detection time, and difficulty in adapting to rapid detection. , to achieve good interspecific specificity, reduced primer number, and good intraspecific conservation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Step (1). Sample pretreatment
[0031] Take 25 g of pork samples from the vegetable market, add 225 mL of sterilized distilled water, and homogenize with a tissue homogenizer for 1 min to obtain a tissue homogenate sample.
[0032] Step (2). Sample DNA extraction
[0033] Take 50 mg of the tissue homogenate sample treated in step (1), add 200 μl of TE buffer solution with a pH value of 8.0, vortex and mix; add 400 μl of lysate, mix well, add 600 μl of phenol-chloroform-isoamyl alcohol mixed solvent, Shake vigorously, centrifuge at 12000g for 10min; take the supernatant, add a mixed solvent of chloroform-isoamyl alcohol equal to the volume of the supernatant, shake vigorously, and centrifuge at 12000g for 10min; take the supernatant, add the same volume of the supernatant Chloroform, shake vigorously, centrifuge at 12000g for 10min; take the supernatant, add isopropanol 0.8 times the volume of the supernatant, and centrifuge at 12000g for 10min; take the precipitate, wa...
Embodiment 2
[0045] Step (1). Sample pretreatment
[0046] Take 25g of fishmeal sample, add 225mL of sterilized distilled water, and homogenize with a tissue homogenizer for 1min to obtain a tissue homogenate sample.
[0047] Step (2). Sample DNA extraction
[0048] Take 50 mg of the tissue homogenate sample treated in step (1), add 200 μl TE buffer solution with a pH value of 8.0, and vortex mix; add 400 μl lysate, mix well, add 600 μl phenol-chloroform-isoamyl alcohol mixed solvent, Shake vigorously, centrifuge at 12000g for 10min; take the supernatant, add a mixed solvent of chloroform-isoamyl alcohol equal to the volume of the supernatant, shake vigorously, and centrifuge at 12000g for 10min; take the supernatant, add the same volume of the supernatant Chloroform, shake vigorously, centrifuge at 12000g for 10min; take the supernatant, add isopropanol 0.8 times the volume of the supernatant, centrifuge at 12000g for 10min; take the precipitate, wash once with ethanol with a volume cont...
Embodiment 3
[0060] Step (1). Sample pretreatment
[0061] Take 25 g of chicken sample from the vegetable market, add 225 mL of sterilized distilled water, and homogenize it with a tissue homogenizer for 1 min to obtain a tissue homogenate sample.
[0062] Step (2). Sample DNA extraction
[0063] Take 50 mg of the tissue homogenate sample treated in step (1), add 200 μl TE buffer solution with a pH value of 8.0, and vortex mix; add 400 μl lysate, mix well, add 600 μl phenol-chloroform-isoamyl alcohol mixed solvent, Shake vigorously, centrifuge at 12000g for 10min; take the supernatant, add a mixed solvent of chloroform-isoamyl alcohol equal to the volume of the supernatant, shake vigorously, and centrifuge at 12000g for 10min; take the supernatant, add the same volume of the supernatant Chloroform, shake vigorously, centrifuge at 12000g for 10min; take the supernatant, add isopropanol 0.8 times the volume of the supernatant, centrifuge at 12000g for 10min; take the precipitate, wash once ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com