Recombinant microorganism producing alkenes from acetyl-COA
A technology for recombining microorganisms and acetylation, applied in the direction of microorganisms, microorganisms, recombinant DNA technology, etc., can solve problems such as inappropriateness and ineffectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
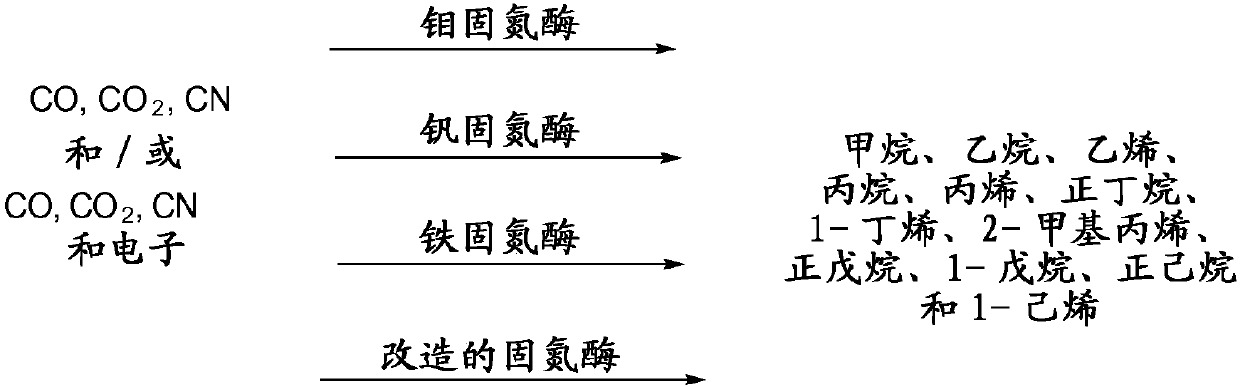
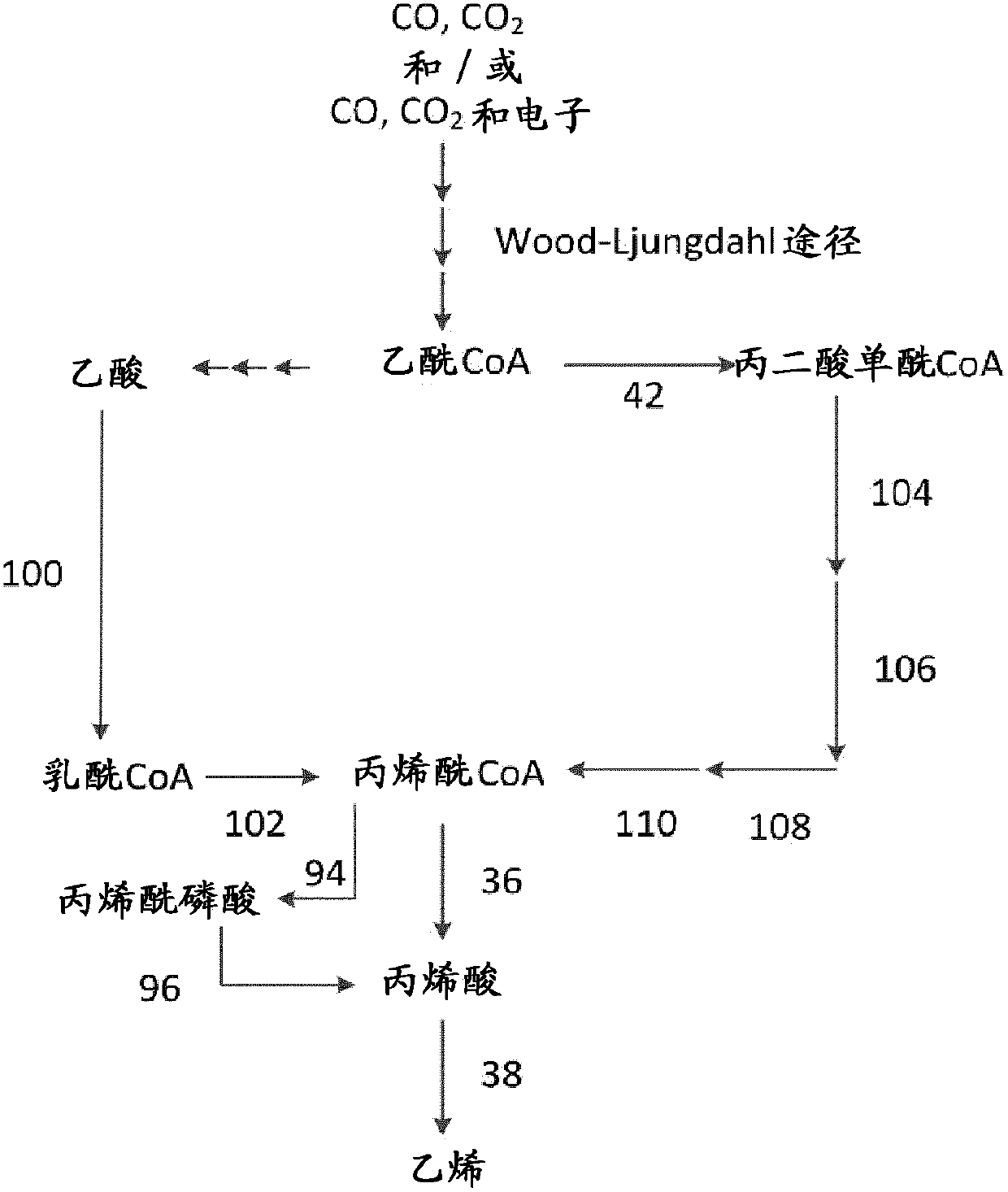
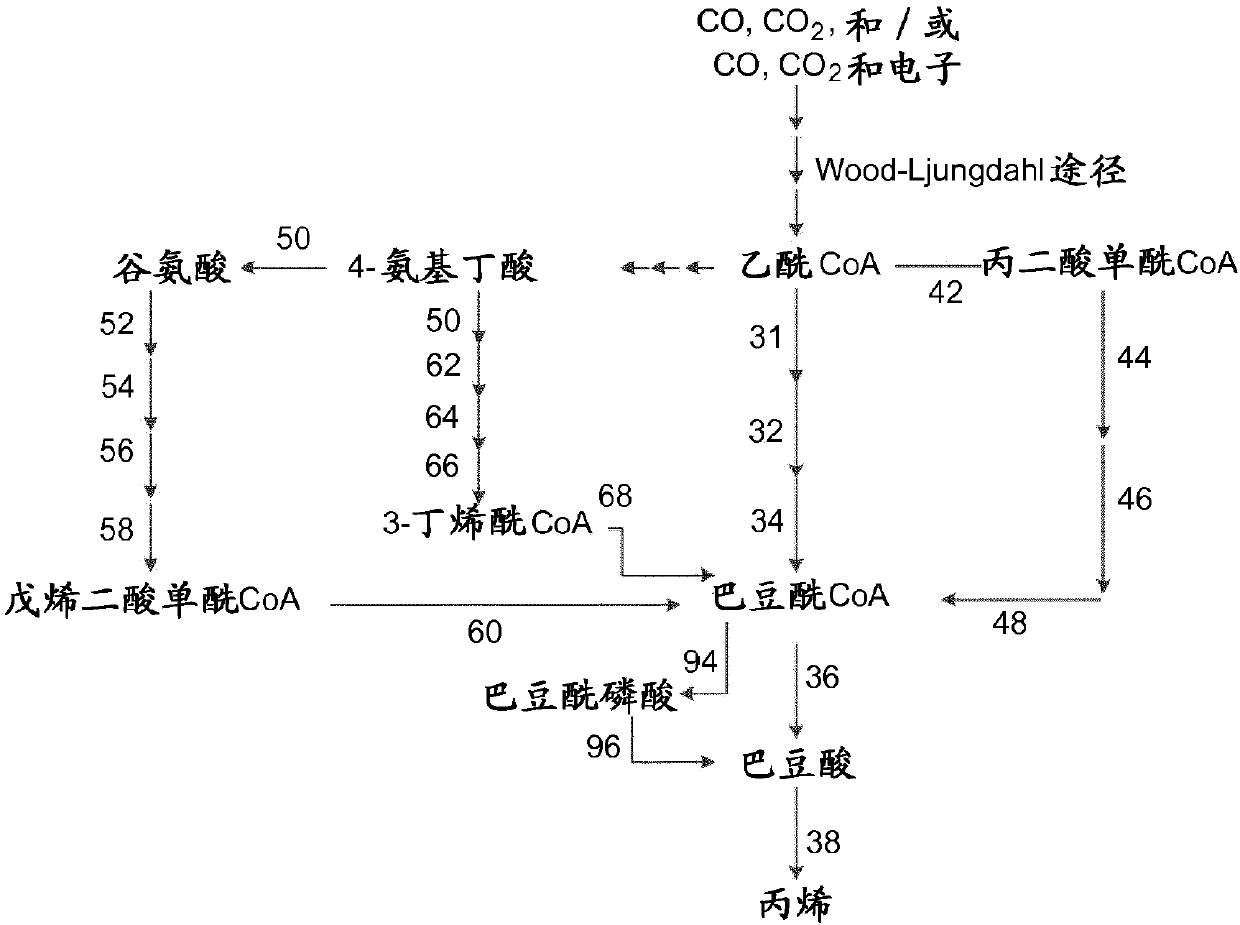
[0488] Example 1: Production of methane, ethane, ethylene, propane, propylene, n-butane, 1-butene, 2-methylpropene, n-pentane, 1-pentene, n-hexane and 1-hexene
[0489] Construction of expression plasmid SG323 (and all other plasmids described herein) was performed by standard recombinant DNA and molecular cloning techniques using genes from Azotobacter vinelandii CA. Figure 4D Plasmid SG323 (SEQ ID No: 4) is shown, which contains the engineered molybdenum nitrogenase pathway. Electroporate this plasmid into C. ljungdahlii by the following procedure:
[0490] Genetic transformation of Clostridium ljungdahlii cells
[0491] Preparation of electrocompetent (electrocompetent) C. ljungdahlii cells: The method for preparing C. ljungdahlii electrocompetent cells was modified from a previously reported procedure ( , M. et al. Proc Natl Acad Sci U S A. (2010) 107, 29, 13087-13092). All manipulations except centrifugation were performed on ice in an anaerobic chamber. All buffers...
Embodiment approach
[0516] Plasmid SG193 ( Figure 4A and SEQ ID NO:1), plasmid SG211 ( Figure 4B and SEQ ID NO:2) and plasmid SG278 ( Figure 4Cand SEQ ID NO:3) were also used to engineer Clostridium ljungdahlii and Clostridium autoethanogenum cells to produce methane, ethane, ethylene, propane, propylene, n-butane, 1-butene, 2-methylpropene, n-pentane , 1-pentene, n-hexane. The same basic scheme and methods are followed as described in the preferred embodiment. Use the different substrates listed below:
[0517] For all substrates used, and those of the subsequent examples:
[0518] 60% CO, 10% CO 2 , 30%H 2
[0519] 100%CO
[0520] 30%CO 2 and 60%H 2
[0521] 60% CO, 10% CO 2 , 30%H 2 and electronics
[0522] 100% CO and Electronics
[0523] 100%CO 2 and electronics
[0524] 30%CO 2 and 60%H 2 and electronics
Embodiment 2
[0525] Example 2: Chromosomal integration via transposases in Clostridium and positive selection for multi-copy chromosomal integration
[0526] The following examples illustrate methods for integrating heterologous DNA (metabolic gene clusters or any other functional or non-functional nucleic acid sequence) into a given host or chassis microorganism. Positive selection (or direct genetic selection) of mutant bacteria is possible when the survival of recombinant bacteria is dependent on the presence or absence of a specific function encoded by the DNA introduced into the organism. An advantage of selection methods over screening methods is that bacteria harboring a particular mutation desired outgrow bacteria lacking the particular mutation greatly, thereby facilitating the identification of preferred mutants. Since all the functions required for transposition (such as the transposase and the integration cassette flanked by the transposition recognition base sequence) are intr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com