Preparation method of activated vitamin D3 drug CD ring intermediate
A technology of vitamins and intermediates, applied in the field of medicinal chemistry, can solve the problems of low total yield and many side reactions, and achieve the effect of less by-products, high product yield and simple process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Synthesis of Diol Derivative 2
[0061]
[0062] Vitamin D 2 (20.0g, 50.4mmol) was dissolved in a mixed solution of 320mL dichloromethane and 80mL methanol, passed through ozone, stirred and reacted at -78°C for 3h, stopped passing through ozone, and passed through N 2 until the blue color disappears. Add NaBH twice 4 (20.0g, 0.528mol), stop refrigeration, electric stirring overnight. Add saturated NH 4 Cl solution, 1N HCL solution quenched the reaction. Extracted several times with dichloromethane, combined organic phases, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, column chromatography (petroleum ether / ethyl acetate=5 / 1) to obtain diol derivatives 2 (white solid) 7.06g, yield 71%.
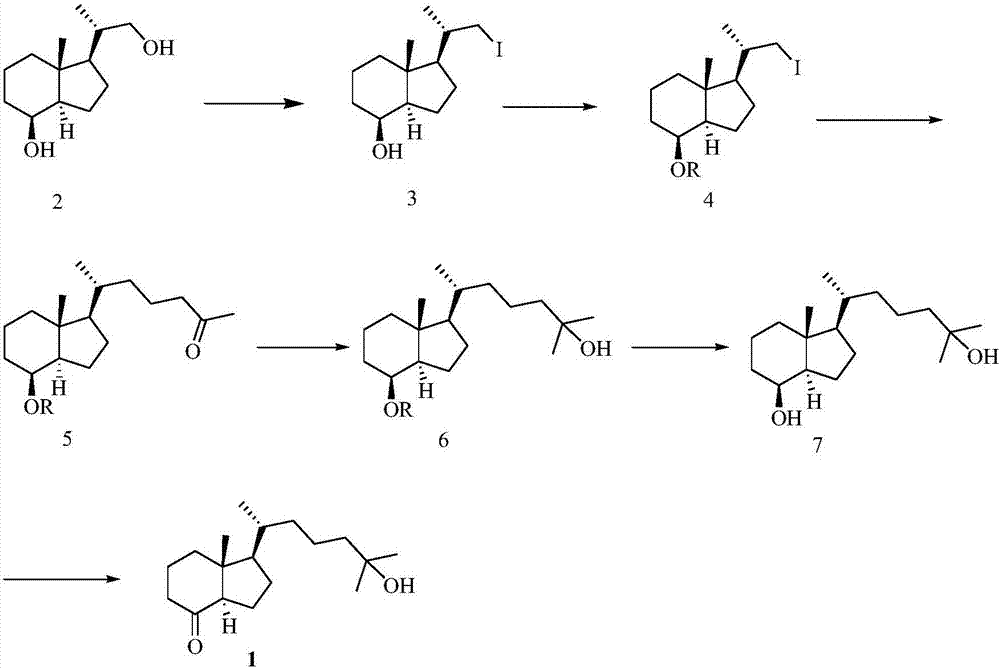
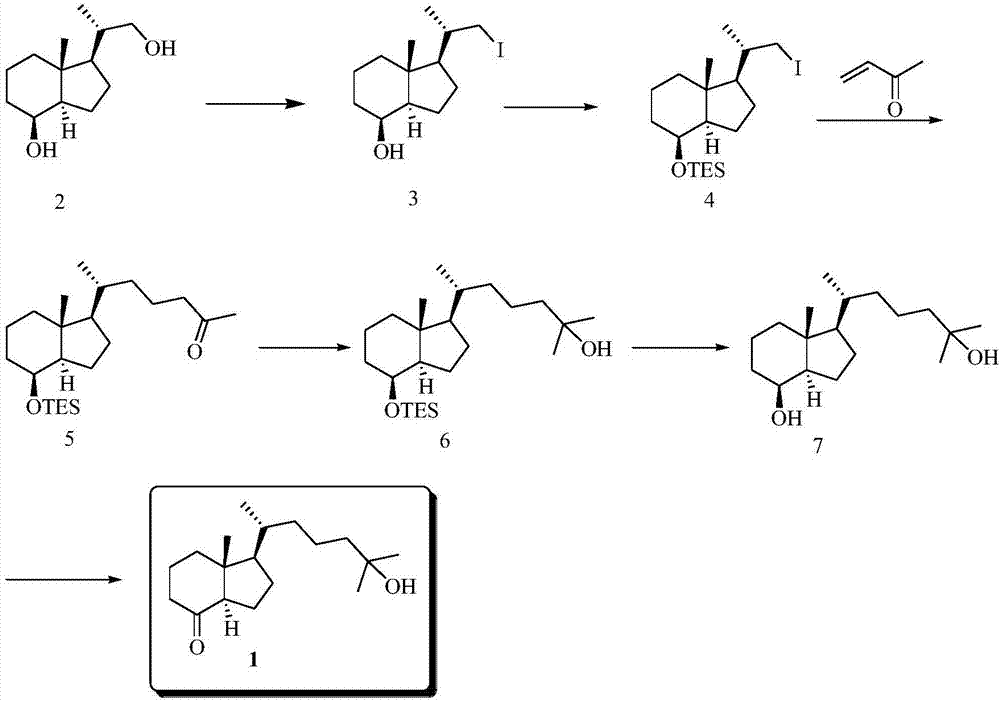
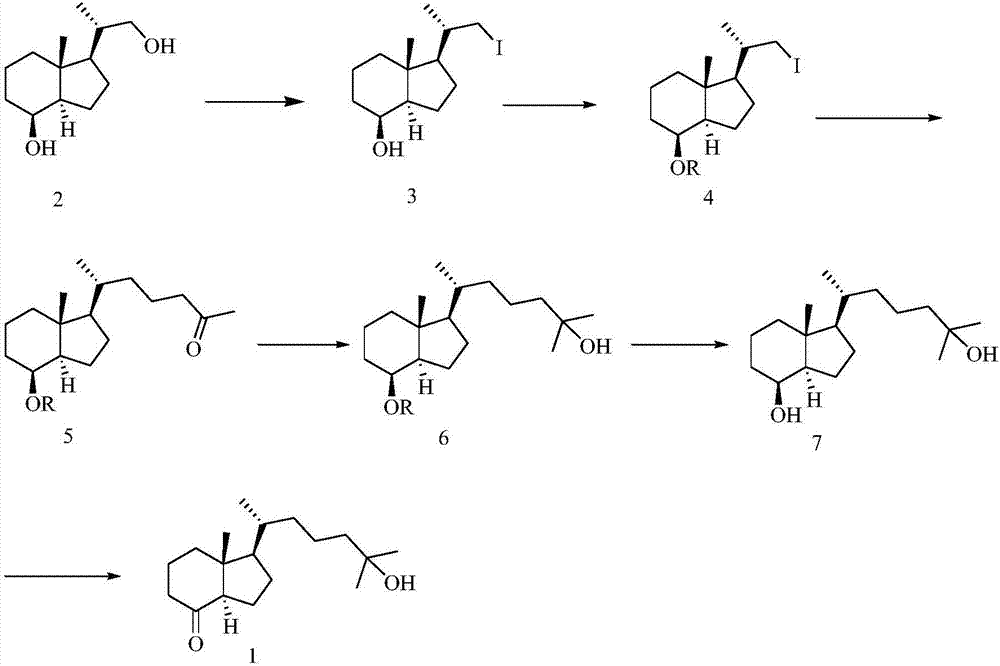
[0063] Preparation of 25-hydroxy Grundmann's ketone from diol derivative 2, such as figure 2 as shown,
[0064] (1) Synthesis of compound 3
[0065]
[0066] Diol derivative 2 (12.4g, 58.4mmol) was dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com