Antigen and antibody of neural recognition molecule contactin 6 and application thereof
A technology for identifying molecules and antibodies, applied in the field of immunology, can solve problems such as lack of pertinence and specificity, lack of axon regeneration antibodies, lack of specificity and affinity of antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of the antigen of neural recognition molecule contactin 6
[0030] Using relevant bioinformatics software (MOE software, Bowtie software, BWA software, Clustalw, etc.), the relevant key sequences of the homologous proteins (contactin-4, contactin-5, contactin-6) of the contactin 6 protein were compared, among which , the key amino acid sequences of contactin-4, contactin-5, and contactin-6 are respectively shown in SEQ ID No.2, SEQ ID No.3, and SEQ ID No.4.
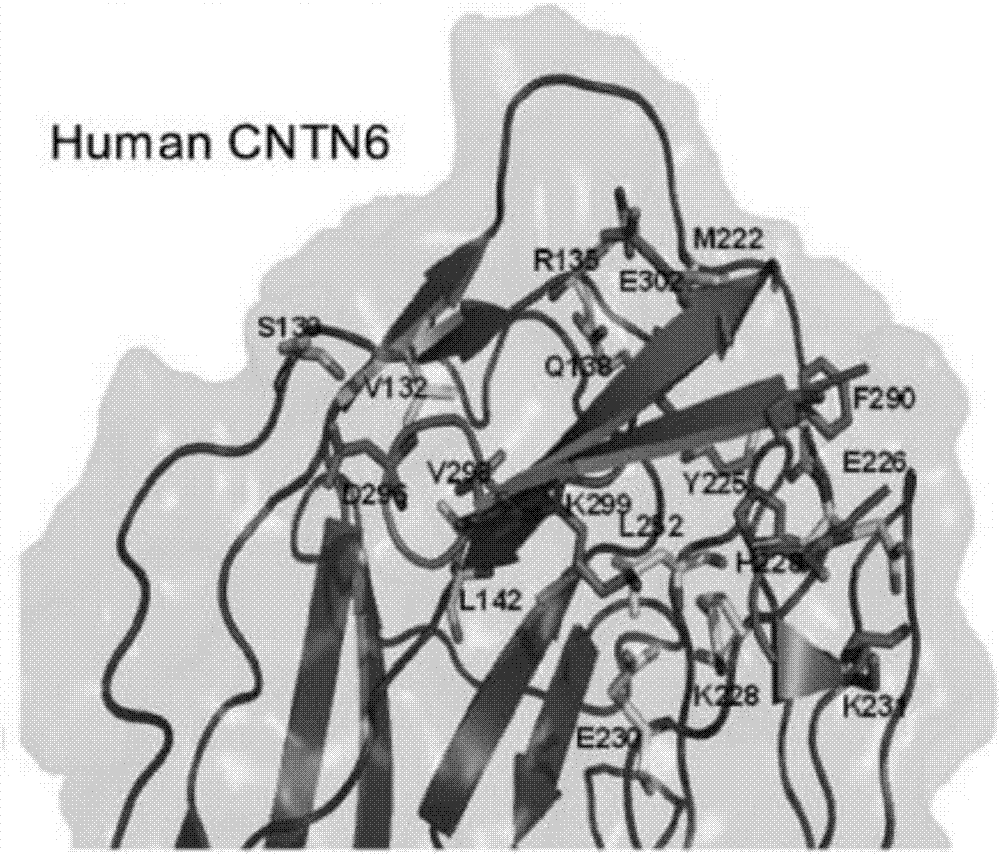
[0031] Using the known crystal structure of the contactin 6FN region, the crystal structure of the IgG region and the PTPR protein complex, in several aspects such as improving the stability of antigen binding, specific binding of the ideal CDR conformation, and conformational changes caused by FR folding, To analyze the effect of antibody design for blocking contactin 6 trans-homologous binding on antibody affinity and specificity.
[0032] Wherein, the corresponding sequence of the IgG 2-3 ...
Embodiment 2
[0038] Example 2 Preparation of antibody to neural recognition molecule contactin 6
[0039]Immunize mice with the above targetedly designed contactin 6 antigen polypeptide, select 6-8-week-old female wild-type mice, let them enter peripheral immune organs through blood circulation or lymphatic circulation, and stimulate corresponding B lymphocyte clones to differentiate into sensitized B lymphocytes. Then the mouse spleen was taken out to prepare a spleen cell suspension, and the myeloma cells and mouse spleen cells were mixed at a ratio of 1:2 to 1:5, and hybridoma cells were formed under the action of polyethylene glycol. In HAT medium, only fused hybridoma cells can obtain hypoxanthine-guanine phosphoribosyltransferase from splenocytes, and have the property of unlimited proliferation of myeloma cells, so they can survive and proliferate in HAT medium. The hybridoma cells were cloned and cultured by the limited dilution method, and the positive hybridoma cells capable of ...
Embodiment 3
[0040] Example 3 Screening and functional testing of antibodies
[0041] (1) Elisa detection
[0042] Spinal cord tissue collection: cut out 5 mm long tissue from the eighth to tenth segment of the thoracic spinal cord tissue centered on the injured area, homogenate the obtained spinal cord tissue, add the tissue to normal saline and mash it, and centrifuge at 3000 rpm for 10 minutes Then take the supernatant. Take out the required strips from the aluminum foil bag after equilibrating at room temperature for 20 minutes, and seal the remaining strips with a ziplock bag and store them at 4°C.
[0043] Set standard wells and sample wells, add 50 μl of IgG solution standard of known concentration to each standard well; add 10 μl of the sample to be tested in the sample well, and add 40 μl of diluent; do not add to the blank well. In addition to the blank wells, add 100 μl of horseradish peroxidase (HRP)-labeled detection antibody to each well of the standard wells and sample wel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com