Double-antibody sandwich ELISA quantitative determination kit of porcine epidemic diarrhea virus and application
A porcine epidemic diarrhea and double-antibody sandwich technology is applied to antiviral immunoglobulins, specific peptides, and measuring devices. It can solve problems such as low sensitivity, inability to quantify, and complicated operations, and achieve good repeatability and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, preparation recombinant N protein
[0029] 1. Sequence Synthesis
[0030] The published N gene sequence of PEDV was optimized according to the codon bias of Escherichia coli to obtain the optimized N gene sequence, as shown in SEQ ID NO:1. The amino acid sequence of N protein is shown in SEQ ID NO:2. The optimized N gene was sent to Gene Company for synthesis, and then the fragment was cloned into the prokaryotic expression vector pET-28a(+) between the BamH I and XhoI restriction sites to obtain the pET-28a-PEDV positive plasmid.
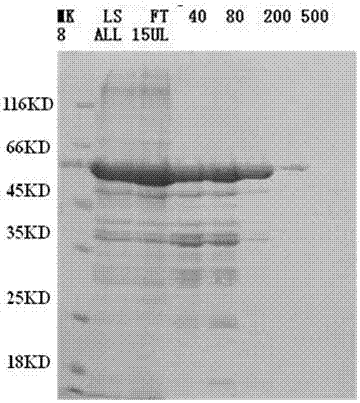
[0031] 2. Expression and purification of recombinant N protein
[0032] (1) Transformation: transform the prokaryotic expression host strain BL21(DE3) with the pET-28a-PEDV positive plasmid, spread it on LB solid medium containing kanamycin (kanamycin concentration 100 μg / mL), and culture at 37°C 12-16h. Pick a single colony and culture it in LB liquid medium containing kanamycin (kanamycin concentration 50μg / mL) for 12-16h...
Embodiment 2
[0037] Embodiment 2, preparation of mouse anti-PEDV N protein monoclonal antibody
[0038] 1. Immunogen preparation: The recombinant N protein (0.5 mg / mL) prepared in Example 1 was mixed with an equal volume of adjuvant to emulsify evenly, and it was in a water-in-oil state to prepare a recombinant N protein vaccine for immunizing mice. The adjuvants used for the first immunization were Freund's complete adjuvant and YOULONG adjuvant, and the adjuvants for the second and subsequent immunizations were Freund's incomplete adjuvant and YOULONG adjuvant.
[0039] 2. Immunization strategy: 4 Balb / c mice were subcutaneously immunized with recombinant N protein vaccine, and each mouse was subcutaneously immunized 3 times, each time each mouse was immunized with 0.1 mg of the immunogen, and the interval between each immunization was 4 weeks. One week after the third immunization, the blood was collected by tail docking, the serum was separated, and the antibody titer was detected by i...
Embodiment 3
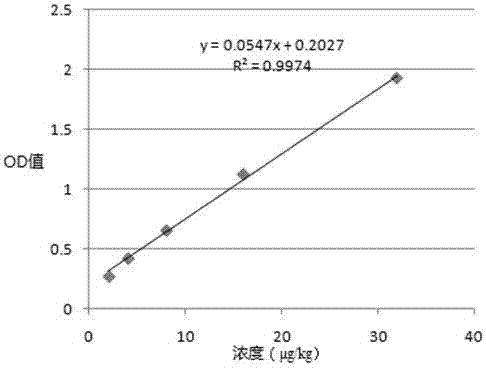
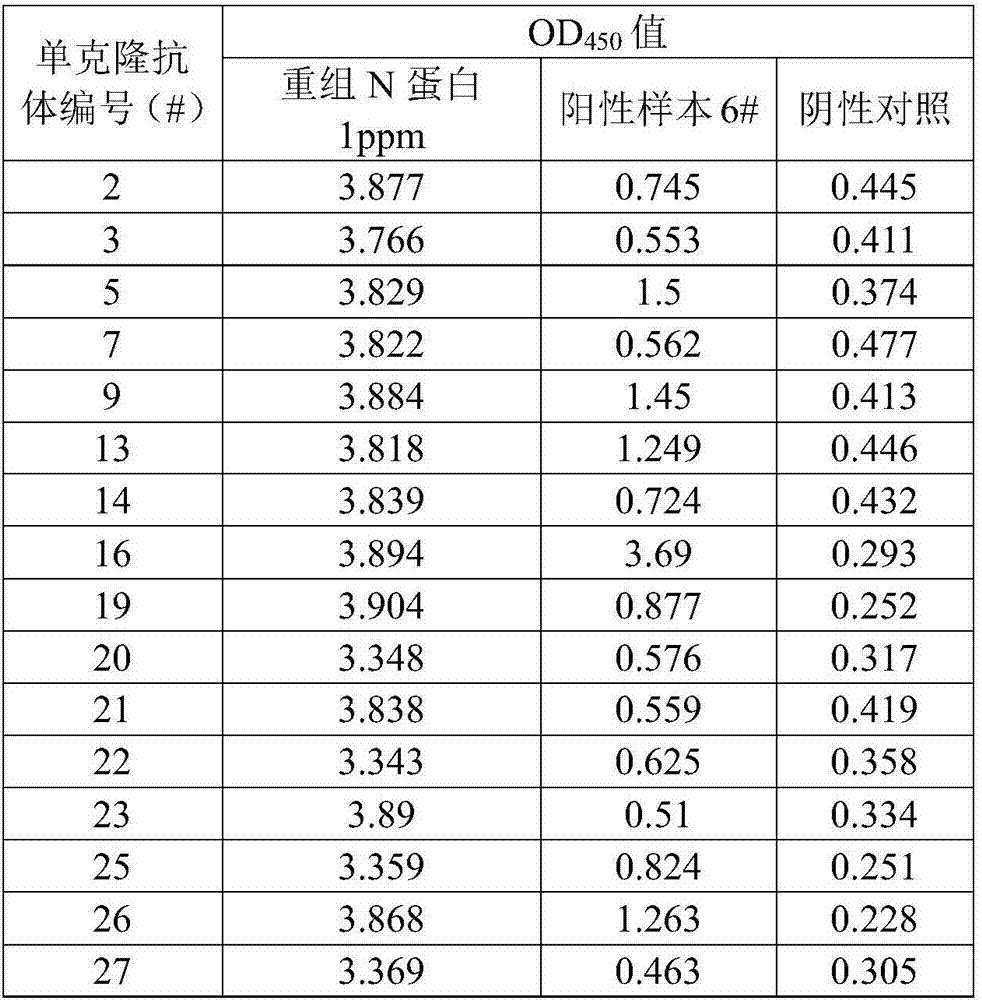
[0065] Example 3, preparation of enzyme-labeled polyclonal antibody and antibody pairing screening
[0066] 1. Preparation of enzyme-labeled polyclonal antibody against porcine epidemic diarrhea virus N protein
[0067] (1) Preparation of polyclonal antibody against porcine epidemic diarrhea virus N protein
[0068] The recombinant N protein prepared in Example 1 was used to immunize rabbits according to conventional methods. When the antibody titer of rabbit serum (detected by the method in Example 2) reached above 1:243000, the rabbit serum was collected.
[0069] The polyclonal antibody was purified by the following method: the rabbit serum was centrifuged at 4000 rpm and room temperature for 15 min, and the supernatant was taken. Slowly add saturated ammonium sulfate solution drop by drop to the supernatant under stirring at 4°C until the saturation of ammonium sulfate in the system reaches 50%, continue stirring for 30 minutes, then centrifuge at 13,000 rpm and 4°C for 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com