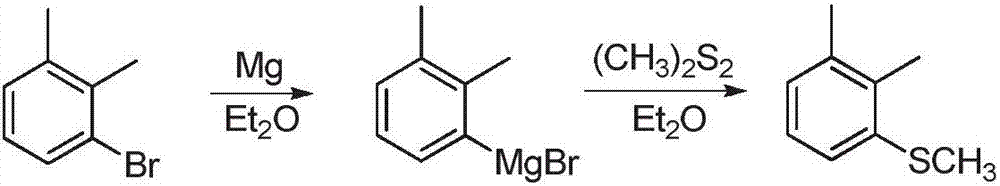
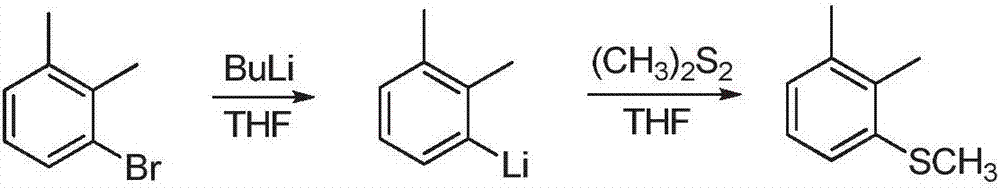
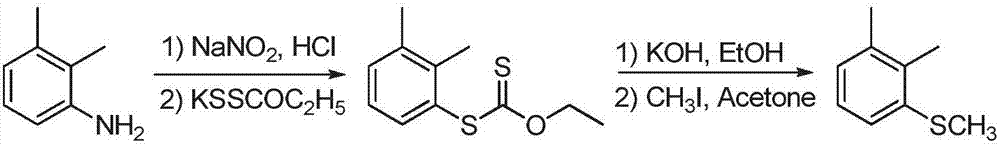
Preparation method of 1,2-Dimethyl-3-methylsulfanyl-benzene
A technology of dimethyl anisole and nitro-o-xylene, applied in the field of preparation, can solve the problems of difficult "three wastes" treatment, high raw material prices, and high production costs, and achieves the advantages of being beneficial to industrialized production and the content of products. High and low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Under nitrogen protection, 61.1g (99%, 0.4mol) 3-nitro-o-xylene, 32.5g (95%, 0.44mol) sodium methyl mercaptide, 0.072g (98%, 0.0004mol) Nickel acetate, 1g (99%, 0.004mol) triphenylphosphine and 427.7g acetone were heated to 50°C and then kept for 20h. After the reaction, add water and toluene, separate the layers, dry the organic phase, and remove the solvent. The residue is rectified in a ceramic random packed tower, and 55.2 g of light yellow liquid 2,3-Di Tolyl sulfide, content 98.2%, yield 89%. 1 H NMR (CDCl 3 , 300MHz) δ: 2.29 (s, 6H, ArCH 3 ×2), 2.44(s, 3H, ArSCH 3 ), 6.97 (dd, J=6.9Hz, J=2.1Hz, 1H, C 6 h 3 -H), 7.03~7.12(m, 2H, C 6 h 2 ).GC-MS (m / z): 152.1 (M + ), 138, 105.1, 77, 45.
Embodiment 2
[0035] Under nitrogen protection, 61.1g (99%, 0.4mol) 3-nitro-o-xylene, 38.4g (95%, 0.52mol) sodium methyl mercaptide, 0.9g (98%, 0.004mol) Nickel bromide, 2.6g (99%, 0.008mol) tetrabutylammonium bromide and 244.4g N,N-dimethylformamide were heated to 70°C and then kept for reaction for 15 hours. After the reaction, add water and toluene, separate the layers, dry the organic phase, and remove the solvent. The residue is rectified in a ceramic random packed tower, and 53.1 g of light yellow liquid 2,3-bis Tolyl sulfide, content 98%, yield 85.5%.
Embodiment 3
[0037] Under nitrogen protection, 61.1g (99%, 0.4mol) 3-nitro-o-xylene, 59g (95%, 0.8mol) sodium methyl mercaptide, 2.6g (99%, 0.02mol) chlorine Nickel chloride, 0.16g (99%, 0.0004mol) methyl trioctyl ammonium chloride and 122.2g N,N-dimethylacetamide were heated to 80°C and then kept for 10h. After the reaction, add water and toluene, separate the layers, dry the organic phase, and remove the solvent. The residue is rectified in a ceramic random packed tower, and 53.4 g of light yellow liquid 2,3-bis Tolyl sulfide, content 98.5%, yield 86.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com