Stimuli-responsive triphenylethylene-based photochromic material, its synthesis method and application
A technology of photochromic materials and tristyrene, which is applied in the directions of color-changing fluorescent materials, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of high material cost, complicated design and synthesis, easy oxidation cycle times, etc. Simple, easy-to-purify effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The synthesis method of the above-mentioned stimuli-responsive triphenylethylene photochromic material comprises:
[0031] Method (1) react a compound containing a phosphine or boron-containing aromatic ring or an aromatic heterocyclic substituent with a triphenylethylene derivative containing halogen at one, two or three terminals to obtain the target product. Preferably, the method (1) is to provide a compound containing a phosphine or a boron-containing aromatic ring or its aromatic heterocyclic substituent and a triphenylethylene derivative containing a halogen, in a toluene solution, under the action of a palladium catalyst, heat and reflux to obtain the target product.
[0032] Method (2) Reaction of benzophenone derivatives with benzene series containing diethyl phosphate groups through Wittig reaction to obtain the target product. Preferably, method (2) is to provide benzophenone derivatives and benzene series containing diethyl phosphate groups in tetrahydrofu...
Embodiment 1
[0037] (1) Synthesis of intermediate [diethyl 4-iodophenylphosphate]
[0038]
[0039] Add 2.95 g of 4-iodobenzyl bromide (0.01 mol) and 6.85 ml of triethyl phosphite (0.04 mol) into a 250 ml single-necked flask. The reaction liquid starts to be a suspension, and on a magnetic stirrer, use a metal heating mantle to heat to 190 ° C Reflux to obtain a clear solution, after reflux for 6 hours, cool to room temperature, distill off excess triethyl phosphite under reduced pressure (oil bath temperature not higher than 120°C), and obtain a solid after cooling the residual solution, wash with n-hexane, filter , and dried to obtain 3.20 g of yellow-white solid with a yield of 90.40%.
[0040] (2) Synthesis of intermediate [1,1-bis(4-chlorobenzene)-2-(4-iodobenzene)ethylene]
[0041]
[0042] Add 4-iodobenzyl bromide (1.77g, 5.00mmol) into a 250mL three-necked flask, add an appropriate amount of THF, and add t-BuOK (1.68g, 15.00mmol) in a nitrogen atmosphere. After stirring for h...
Embodiment 2
[0047]
[0048] The product obtained in Example 1 (0.38 g, 0.75 mmol) was added into a round bottom flask, and dissolved in 20 mL of tetrahydrofuran. 6 mL of aqueous hydrogen peroxide solution (30%) was added, and after stirring for 5 hours, 50 mL of dichloromethane and 50 mL of water were added to the reaction liquid, followed by liquid separation. The dichloromethane layer was spin-dried with a rotary evaporator to obtain a white powder. The white powder was recrystallized from dichloromethane / n-hexane to obtain 0.34 g of white solid with a yield of 86.28%. 1 H NMR (600MHz, CDCl 3 ,298K,relative toMe 4 Si): δ=6.9599(s,1H),7.10-7.14(m,4H),7.24(d,8.6Hz,2H),7.31-7.33(m,4H),7.45-7.50(m,6H),7.55 -7.58(m,2H),7.65-7.68(m,4H).
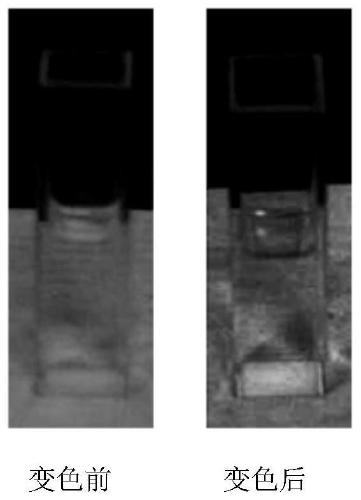
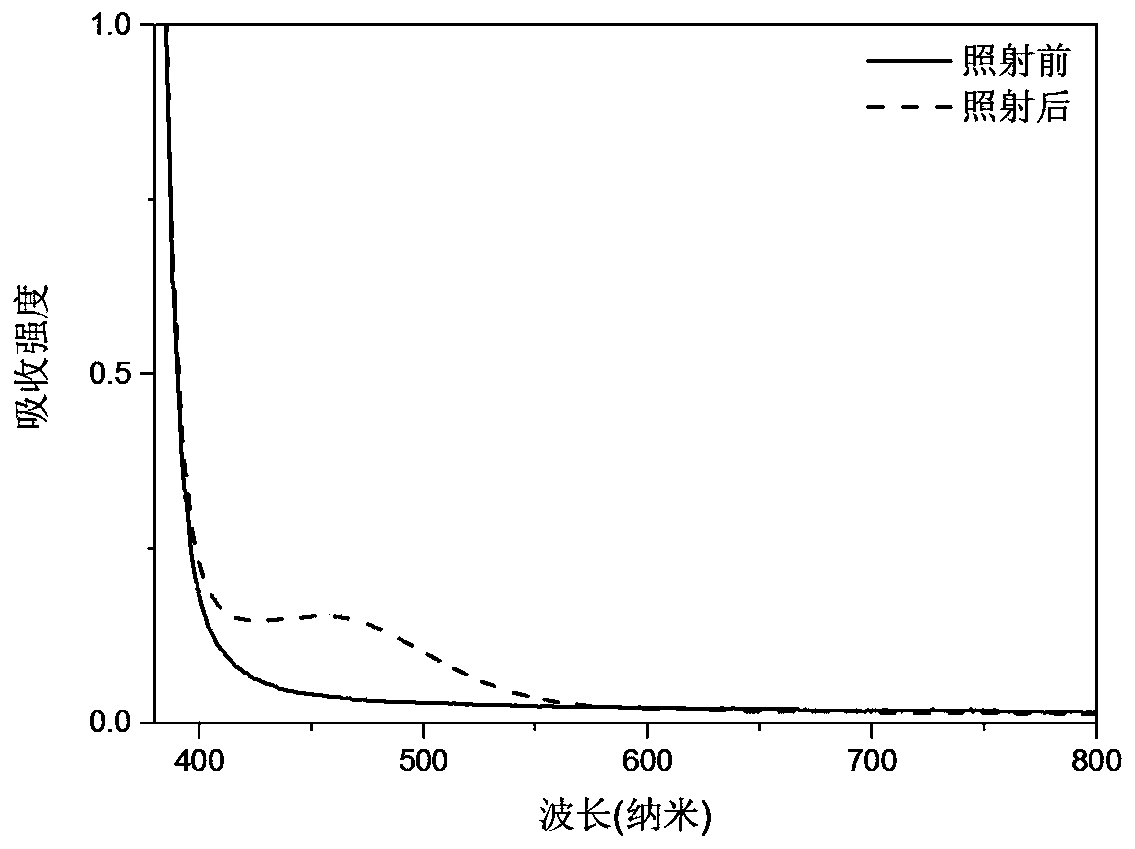
[0049] The comparison before and after the solution photochromism of the product of this example figure 1 As shown, the UV absorption spectrum is compared with figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com