The preparation method of high ortho bisphenol f
A high-ortho-position and resin technology, applied in the preparation of organic compounds, organic chemical methods, chemical instruments and methods, etc., can solve the problems of complex post-treatment process, equipment corrosion environment, low yield, etc., to avoid equipment corrosion, Inexpensive, easy post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
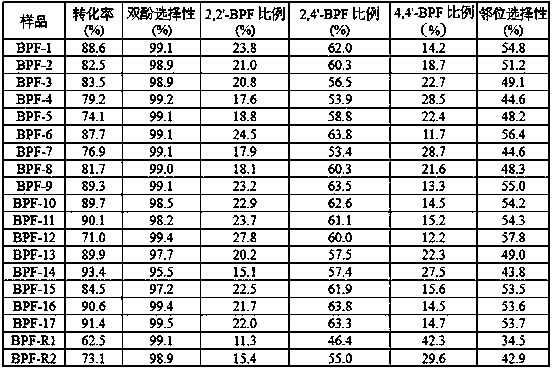
[0020] Modification of resin catalyst: Place 50 mL of commercially available D005 resin catalyst in 50 mL of dilute hydrochloric acid with a concentration of 1 mol / L, soak at room temperature for 8 hours, wash with deionization to neutrality, and suction filter to obtain H-type cationic resin; 1mol / L Zn(OAc) 2 Slowly add 100 mL of aqueous solution into 50 mL of H-type cationic resin, react at room temperature for 1 h, wash with deionized water three times, and filter with suction to obtain a modified cationic resin catalyst.
[0021] Synthesis of bisphenol F: Take 16mL of modified cationic resin catalyst and put it in a three-necked flask, add 16.2g of 37wt% formaldehyde aqueous solution (0.2mol), stir at 30°C for 1h; add 94g of phenol (1mol) and stir well, then heat up to 70°C After reacting for 5 h, the catalyst was separated by filtration, and the filtrate was distilled under reduced pressure to remove water and recover phenol. After cooling, the bisphenol F product was obt...
Embodiment 2
[0023] According to the method of embodiment 1, only with Mg(OAc) 2 solution instead of Zn(OAc) 2 The solution modifies the H-type cation exchange resin, and the final bisphenol F product obtained is denoted as BPF-2.
Embodiment 3
[0025] According to the method of embodiment 1, only with Mn(OAc) 2 solution instead of Zn(OAc) 2 The solution modifies the H-type cation exchange resin, and the final bisphenol F product obtained is denoted as BPF-3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com