Preparation method of high-purity (±)-2,2'-bis-(2-hydroxyethoxy)-1,1'-binaphthyl
A hydroxyethoxylated, high-purity technology, applied in ether preparation, ester reaction to ether, organic chemistry, etc., can solve the problems of many side reactions, long reaction time, high reaction temperature, etc., to simplify post-processing steps and suppress side effects The formation of product polymers and the effect of improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
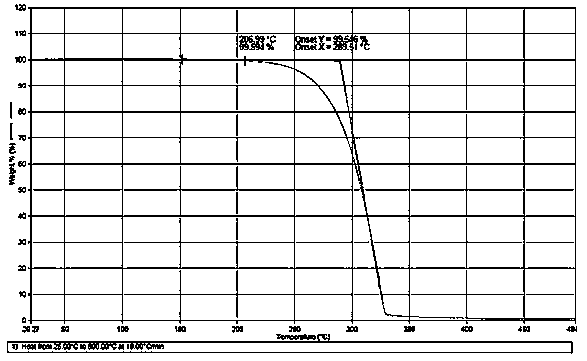
[0027] In a 500mL four-neck flask, add (±)-1,1'-bi(2-naphthol) 28.6g (0.1mol), 50g toluene, 4g N,N-dimethylacetamide, 1.9g potassium carbonate and Ethylene carbonate 22g (0.25mol). at 100 o C After 6 hours of reaction, the content of (±)-2,2'-bis-(2-hydroxyethoxy)-1,1'-binaphthyl was less than 0.1% by HPLC analysis, and the reaction was stopped. Add toluene, wash with water until neutral, cool to precipitate a solid, and vacuum dry for 12 hours to obtain (±)-2,2'-di-(2-hydroxyethoxy)-1,1'-binaphthalene 31.8g , the yield was 84.9%, the analytical purity by high performance liquid chromatography (HPLC) was 99.2%, and the melting endothermic peak range was 105-116 o C ( figure 1 ), the weight loss on drying at 120°C was 0.10%, and the TGA thermal analysis at 200°C lost 0.4% ( figure 2 ); 1 HNMR (CD 3 SOCD 3 ) δ: 3.86(t,4H), 4.03(t,4H), 6.90(d,2H), 7.23(d,2H), 7.34(dd,2H), 7.60(dd,2H), 7.92(d,2H ), 8.06 (d, 2H), 4.58 (s, 2H, plus D 2 O disappears).
Embodiment 2
[0029] In a 500mL four-neck flask, add (±)-1,1'-bi(2-naphthol) 28.6g (0.1mol), 50g toluene, 3g N,N-dimethylformamide, 1.9g potassium carbonate and Ethylene carbonate 22g (0.25mol). at 100 o C was reacted for 6 hours, and the content of (±)-1,1'-bi(2-naphthol) was less than 0.1% according to HPLC analysis, and the reaction was stopped. Add toluene, wash with water until neutral, cool to precipitate a solid, and vacuum dry for 12 hours to obtain (±)-2,2'-di-(2-hydroxyethoxy)-1,1'-binaphthalene 31.2g , the yield was 83.3%, the analytical purity by high performance liquid chromatography (HPLC) was 99.3%, and the melting endothermic peak range was 105-116 o C ( figure 1 ).
Embodiment 3
[0031] In a 1000mL four-neck flask, add (±)-1,1'-bi(2-naphthol) 114.4g (0.4mol), 100g toluene, 6gN,N-dimethylformamide, 7.5g potassium carbonate and carbonic acid Vinyl ester 88g (1mol). at 100 o C was reacted for 6 hours, and the content of (±)-1,1'-bi(2-naphthol) was less than 0.1% by HPLC analysis, and the reaction was stopped. Add toluene, wash with water until neutral, cool to precipitate a solid, and vacuum dry for 12 hours to obtain (±)-2,2'-di-(2-hydroxyethoxy)-1,1'-binaphthalene 125.8g , the yield was 84.1%, the analytical purity by high performance liquid chromatography (HPLC) was 99.3%, and the melting endothermic peak range was 105-116 o C ( figure 1 ), 120 o The loss on drying under C condition is 0.15%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com