Oral targeting nanoparticles based on intestinal epithelial cell apical OCTN2 transporter
A nanoparticle and stearyl technology, which is applied in the application field of oral drug carrier in drug delivery, can solve the problem that anti-tumor drugs cannot be taken orally, and achieve the effect of easy operation, good stability and high encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
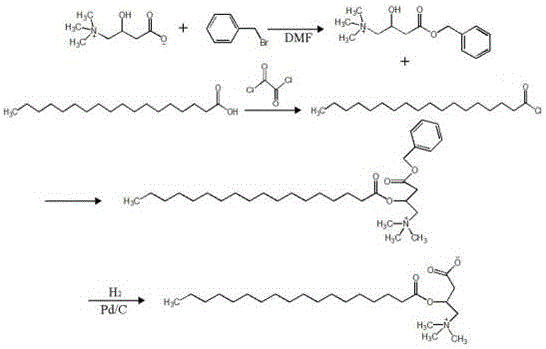
[0049] Synthesis of Targeting Modifier L-Carnitine Derivatives
[0050] Add 3mmol (about 484mg) of L-carnitine and 3.6mmol of benzyl bromide into about 25ml of DMF, stir evenly, heat to 125°C, react under stirring conditions for 4 hours, distill off the solvent DMF and unreacted benzyl bromide under reduced pressure , That is, L-carnitine benzyl ester. Add 3mmol of stearic acid into about 15ml of dichloromethane and stir to dissolve, add 3.6mmol of oxalyl chloride dropwise, react for 0.5h under stirring conditions, distill off the solvent dichloromethane and unreacted oxalyl chloride under reduced pressure to obtain stearin acid chloride. Add the L-carnitine benzyl ester and stearyl chloride obtained above into about 25ml of acetonitrile and stir evenly, heat to 45°C, react under stirring conditions for 24h, remove the acetonitrile under reduced pressure, and use dichloromethane:methanol=19:1 The mobile phase is subjected to column separation to obtain stearoyl-L-carnitine b...
Embodiment 2
[0053] Preparation of ordinary PLGA NPs
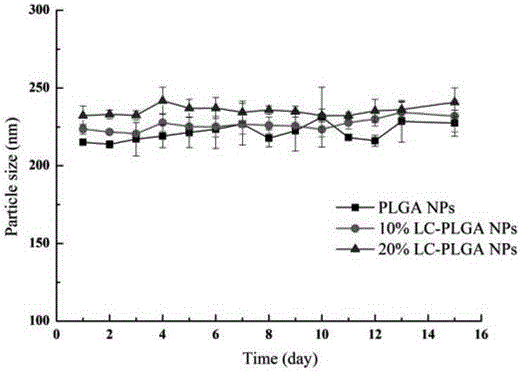
[0054] Accurately weigh 0.50mg of paclitaxel (or coumarin 6), 10.0mg of PLGA, dissolve in 1mL of dichloromethane, mix it with 5mL of 1% PVA aqueous solution, sonicate for 5min with a power probe of 200w, stir at room temperature for 5h, The organic solvent was evaporated to obtain a nanoparticle solution. After centrifugation at 13000r / min for 30min, the supernatant was discarded, and deionized water was added to disperse. The operation was repeated three times, and the surfactant was washed away to obtain ordinary unmodified PLGA NPs.
Embodiment 3
[0056] Preparation of 5% LC-PLGA NPs
[0057] Accurately weigh 0.50 mg of paclitaxel (or coumarin 6), 0.50 mg of synthetic stearoyl-L-carnitine, and 10.0 mg of PLGA, dissolve them in 1 mL of dichloromethane, and mix them with 5 mL of 1% PVA aqueous solution Mix, ultrasonicate with 200w power probe for 5min, stir at room temperature for 5h, evaporate the organic solvent to obtain a nanoparticle solution, centrifuge at 13000r / min for 30min, discard the supernatant, add deionized water to disperse, repeat the operation three times, and wash off the surface active agent, that is, 5% LC-PLGA NPs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com