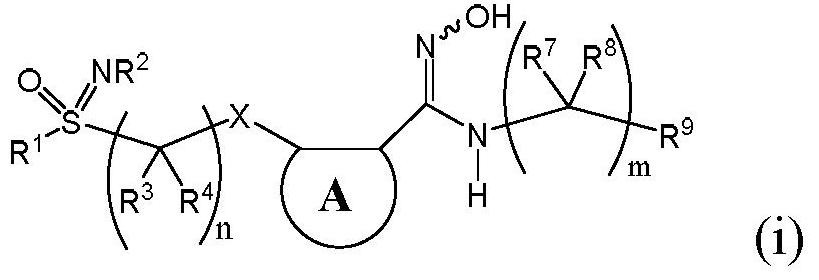
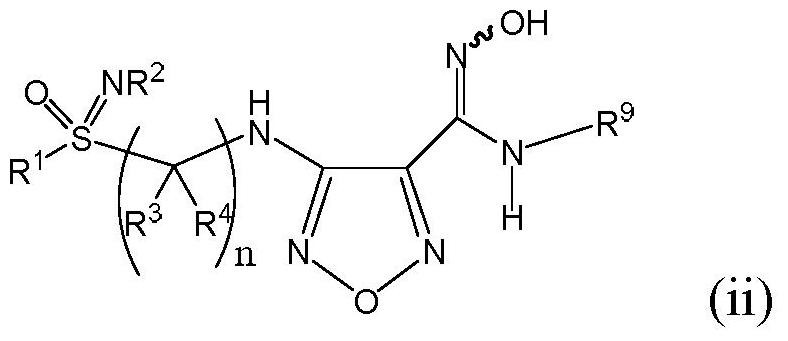
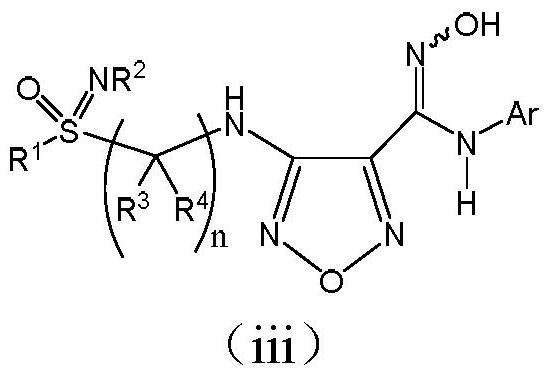
An indoleamine-2,3-dioxygenase inhibitor of nitrogen-containing alkylated and arylated sulfoximines
A technology of alkyl and aryl, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0252] (±)(Z)-nitrogen-(3-bromo-4-fluorophenyl)-nitrogen'-hydroxy-4-((2-(nitrogen,sulfur-dimethylsulfoximine)ethyl)amino) -1,2,5-oxadiazole-3-carboxamidine
[0253]
[0254] The first step: 4-amino-nitrogen'-hydroxy-1,2,5-oxadiazole-3-carboxamidine
[0255]
[0256] Dissolve malondicyanide (3.2g, 48.5mmol) in 70mL of water and heat until completely dissolved. Under cooling in an ice-water bath, sodium nitrite (3.8 g, 55 mmol) and 6N hydrochloric acid (0.6 mL) were added. After reacting in an ice bath for 0.5 hours, the temperature was raised to room temperature and reacted for 2 hours. The reaction solution was continued to be cooled in an ice bath, and 50% aqueous solution of hydroxylamine hydrochloride (9.9 g, 150 mmol) was added dropwise to the reaction solution. After stirring for half an hour, the reaction solution was raised to room temperature for 1 hour. Heat to reflux for 2 hours. After the reaction is complete, adjust the pH to 7.0 with 8 mL of 6N hydrochlor...
Embodiment 2
[0301] (±)(Z)-nitrogen-(3-bromo-4-fluorophenyl)-nitrogen'-hydroxy-4-((3-(sulfur-methyl-nitroethylsulfoximine)ethyl)amino )-1,2,5-oxadiazole-3-carboxamidine
[0302]
[0303] According to the preparation method of Example 1, the reagent in the tenth step was replaced with triethyloxonium tetrafluoroborate, and then the conditions in the eleventh step were used to obtain the target compound.
[0304] 1 H NMR: (400MHz, acetone-d 6 ):δ10.85(s,1H),8.12(s,1H),7.28-7.26(m,1H),7.17-7.13(m,1H),7.02-6.98(m,1H),6.64(s,1H ),3.81(s,2H),3.57(s,1H),3.43-3.40(m,1H),3.31-3.06(m,5H),1.11-1.05(m,3H).
[0305] MS(ESI):[M+H] + m / z=449.0
Embodiment 3
[0307] (±)(Z)-nitrogen-(3-bromo-4-fluorophenyl)-nitrogen'-hydroxy-4-(2-(sulfur-methyl-nitrogen-phenylsulfoximine)ethyl)amino )-1,2,5-oxadiazole-3-carboxamidine
[0308]
[0309] The first step: (±) 4-(3-bromo-4-fluorophenyl) 3-4-(((2-(methyl sulfoxide phenylimine) ethyl) tert-butoxycarbonylamino)-1 ,2,5-oxadiazol-3-yl)-1,2,4-oxadiazolone
[0310]
[0311] The product of the ninth step of Example 1 (20mg, 0.037mmol) was dissolved in dichloromethane (2ml), added phenylboronic acid (8mg, 0.055mmol), copper acetate (5mg) and stirred at room temperature for 2 hours, TLC showed that the reaction was complete , water (10ml), ethyl acetate (10mL) were added to the reaction solution, and then extracted with ethyl acetate (2x 10mL) to combine the organic phases, then washed with water (10mL), washed with saturated brine (10mL), and dried over sodium sulfate. After concentration, separation and purification by column chromatography gave the target compound (15 mg, 66% yield)
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com