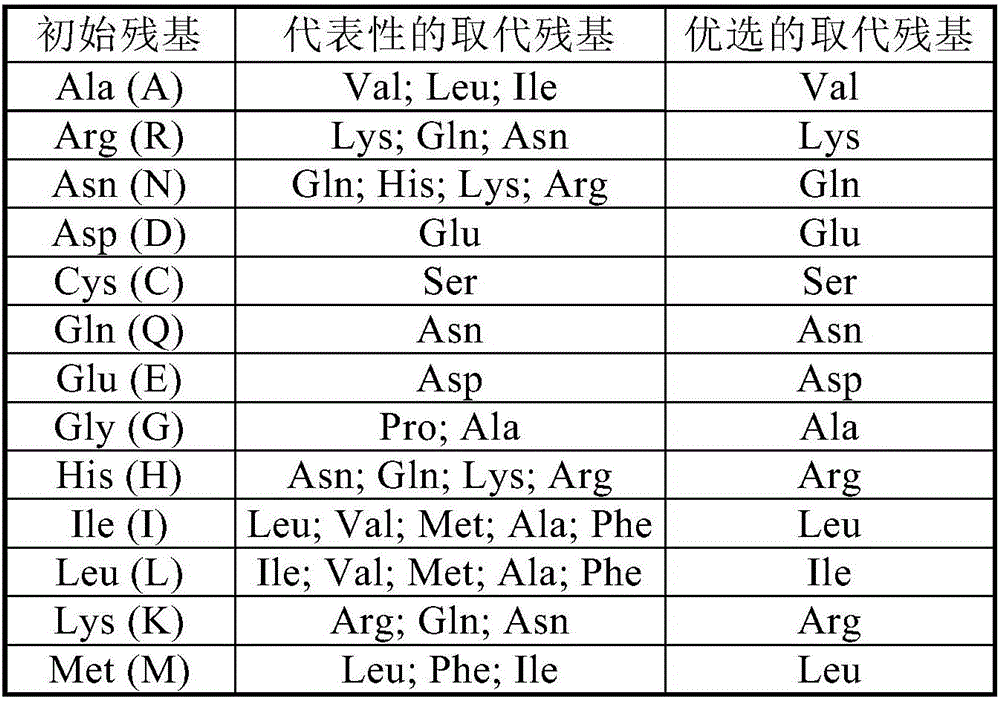
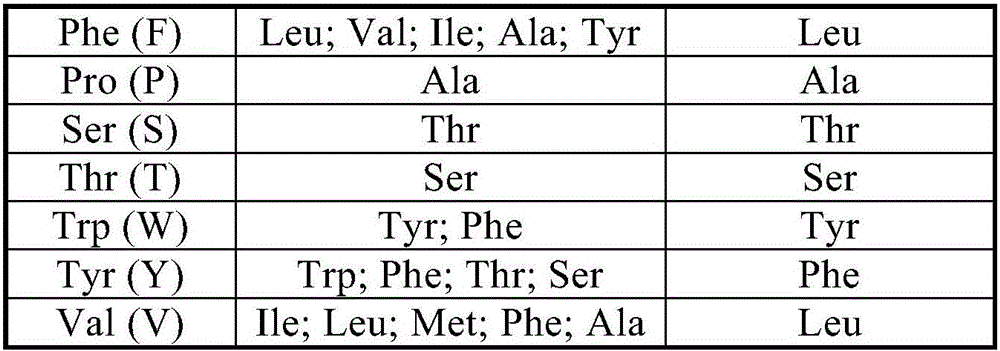
Novel lysine decarboxylase and application thereof
An amino acid, application technology, applied in the biological field, can solve problems such as poor thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1. Expression plasmid and bacterial strain construction of lysine decarboxylase
[0101] The lysine decarboxylase sequence of the present invention is shown in the following SEQ ID NO: 1-2:
[0102] SEQ ID NO:1(命名为Ldc6)–MNIFAILNHSGVFFKEEPVRELHASLEKAGYKVVYPVDAQDLYKMVEMNPRICGVLFDWDKYSLDLCTEINVLNEKLPLYAFANQHSTLDISLTDLRLNLHFFEYALGMADDIALKINQATEEYIDQIMPPFTKALFKYVEEGKYTFCTPGHMGGTAFQKSPAGSIFYDFYGPNAFKADVSISMPELGSLLDHSGPHKEAEEYIARTFNADSSYIVTNGTSTSNKIVGMFSAPAGSTVLVDRNCHKSLTHMMMMSDVTPIYFRPTRNAYGILGGIPQSEFTREVIEAKVAATPNATMPGYAVITNSTYDGLLYNTQYIKETLDTKFIHFDSAWVPYTNFNSIYEGKCGMSGEAMPGKVFYETQSTHKLLAAFSQASMIHVKGEFDKESFNEAFMMHTSTSPQYGIVASTEIAAAMMRGNTGKKLIQDSIDRAIRFRKEIKRLESESDSWFFDVWQPENIDTTECWKLDPKDTWHGFKDIDDDHMYLDPIKVTLLTPGMNENSEMSETGIPASIVAKYLDEHGIVVEKTGPYNLLFLFSIGIDKSKAMQLLRALTDFKRGYDLNLTVKNFLPSLYNEDPSFYEGMRIQELAQGIHDLTRQYRLPELMFKAFDVLPELKVTPHAAWQEELRGNVEEVKLEEMVGRVSANMILPYPPGVPLVLPGEMVTTESRPVLDFLEMLCAIGAHYPGFETDIHGVYAQKDGSYTVKVLKED;
[0103] SEQ ID NO:2(命名为Ldc14)–MKDILFLCNPSP...
Embodiment 2
[0105] Embodiment 2. Separation and purification of enzyme
[0106] The recombinant bacteria SKEcC2, SKEcC3 and SKEcC4 were respectively inserted into 5 mL LB liquid medium containing 100 μg / mL ampicillin, and cultured at 37 °C and 200 r / min for about 10 h. Take 0.5mL of seed solution and put it into a 500mL shake flask containing 100μg / mL ampicillin in 50mL LB liquid medium, and culture at 37°C and 200r / min. When grown to OD 600 When it is 0.6-0.8, add ITPG to a final concentration of 0.1 mM, induce expression at 20°C, 200r / min for 20h. The induced bacterial solution was centrifuged at 4°C to collect the bacterial cells, and the 4°C pre-cooled resuspension buffer (20mM potassium phosphate, 20mM imidazole, 0.1mM pyridoxal phosphate, 1mM DTT, 100mM NaCl, adjusted to pH=7.4) was used for induction After the cells were resuspended and washed 3 times, they were disrupted by ultrasound. The broken solution was centrifuged at 12,000rpm for 20min, and the obtained soluble supernat...
Embodiment 3
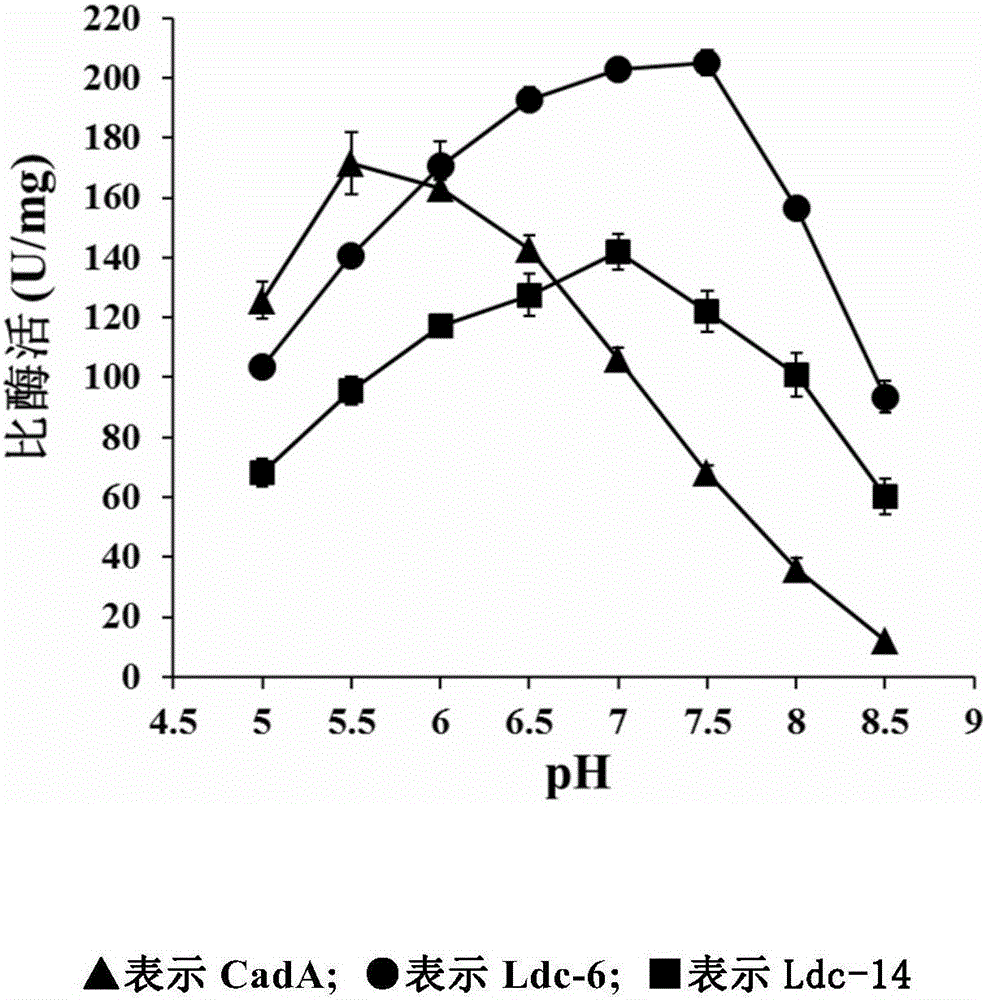
[0107] Embodiment 3. Different pH conditions enzyme activity assay
[0108] In a 100ul enzyme reaction system (100mM potassium phosphate, 0.1mM pyridoxal phosphate, 1mM DTT, 100mM NaCl) the amount of LDC pure enzyme is 0.5ug, the concentration of substrate L-lysine is 15mM, and the pH of the buffer solution is adjusted to 5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, react at 37°C for 3 minutes. Take 30ul of the reaction solution and add it to 70ul of 1M sodium carbonate stop solution to stop the reaction, take 50ul of 10mM TNBS into the reaction stop solution, develop color at 42°C for 5min, and finally extract with 500ul of toluene, draw 200μL of the supernatant on the microplate reader Read the absorbance at 340nm. The enzyme activity unit U is defined as: under the above enzyme reaction conditions, the amount of enzyme required to catalyze the production of 1 μmol 1,5-pentanediamine per minute is defined as an enzyme activity unit.
[0109] The specific enzyme activities of Ldc6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com