Method for synthesizing VIII first-row transition metal and molybdenum/tungsten double-metal carbide catalyst at low temperature
A bimetallic carbide and bimetallic oxide technology, applied in physical/chemical process catalysts, chemical instruments and methods, organic chemistry, etc., can solve the problems of bimetallic carbide carbon pollution, block active sites, etc., and achieve operational Simple, energy-saving product particles, the effect of less surface carbon pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
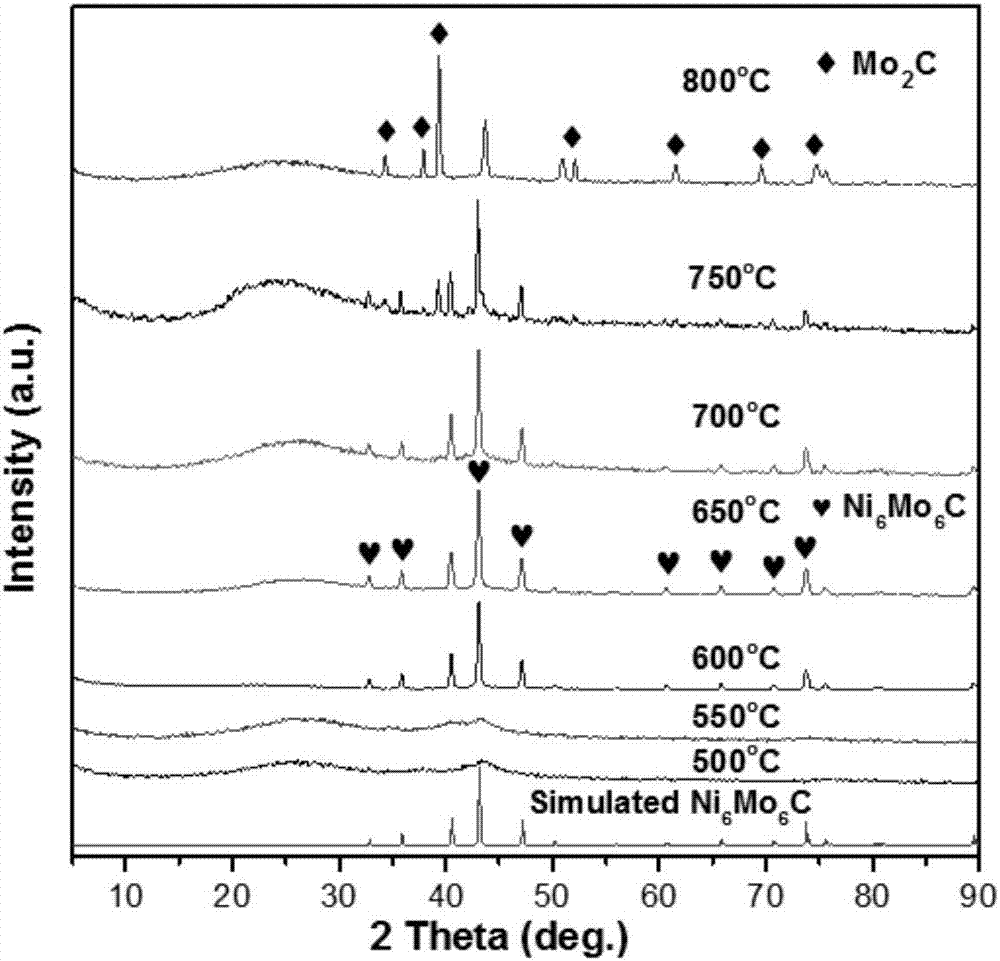
[0026] Embodiment 1: the Na of 0.005mol 2 MoO 4 2H 2 O and 0.005mol Ni(NO 3 ) 2 ·6H 2 O was dissolved in 20 mL deionized water, respectively, and stirred to mix at room temperature. A light green precipitate was obtained, and after stirring for 30 minutes, the mixture was transferred and sealed in a 60 mL polytetrafluoroethylene-lined stainless steel autoclave, and kept in an electric oven at 180 °C for 4 hours. Then the autoclave was taken out from the oven and allowed to cool to room temperature naturally. The yellow-green precipitate was collected by centrifugation, washed well with water and ethanol, and dried overnight at 80 °C. Finally, the product was ground into a fine powder for characterization.
[0027] Activated carbon (AC) was prepared through a chemical activation route from coconut shells. The specific surface area of activated carbon was obtained by the nitrogen adsorption and desorption isotherm measurements at 77 K to be about 1000 m 2 / g, the aver...
Embodiment 2
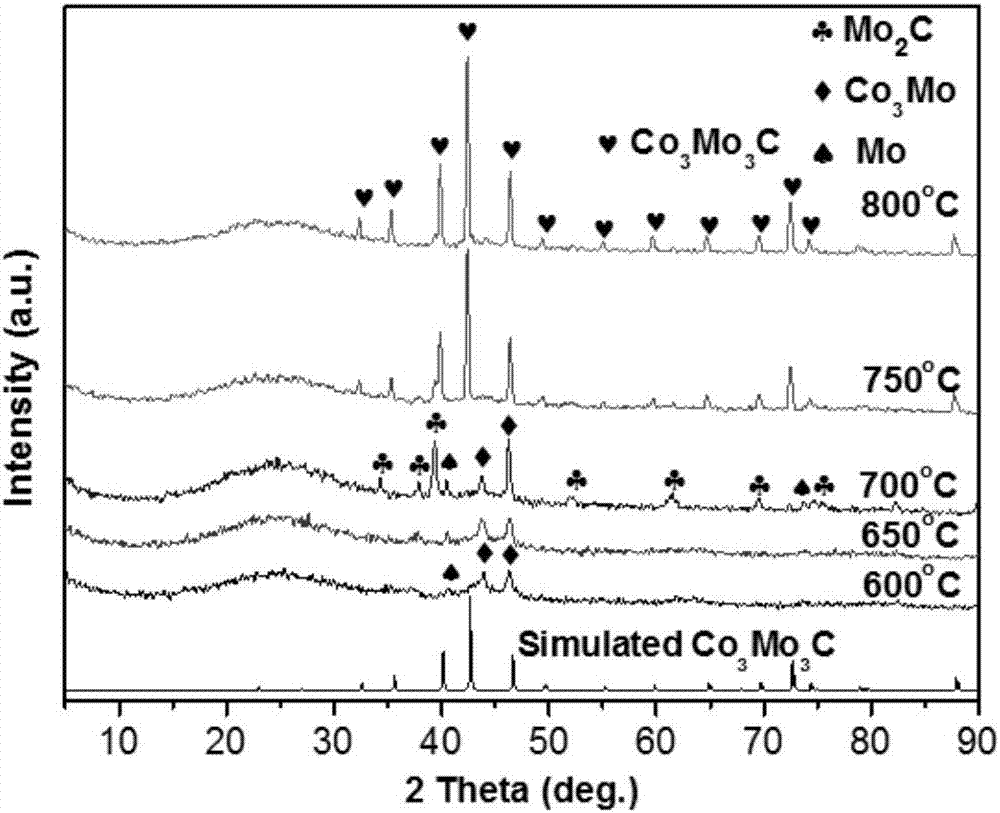
[0028] Embodiment 2: the Na of 0.005mol 2 MoO 4 2H 2 O and 0.005mol of Co(NO 3 ) 2 ·6H 2 O was dissolved in 20 mL deionized water, respectively, and stirred to mix at room temperature. A light green precipitate was obtained, and after stirring for 30 minutes, the mixture was transferred and sealed in a 60 mL polytetrafluoroethylene-lined stainless steel autoclave, and kept in an electric oven at 180 °C for 4 hours. Then the autoclave was taken out from the oven and allowed to cool to room temperature naturally. The yellow-green precipitate was collected by centrifugation, washed well with water and ethanol, and dried overnight at 80 °C. Finally, the product was ground into a fine powder for characterization.
[0029] The as-synthesized bimetallic oxide was mixed with activated carbon so that the molar ratio of total metal to carbon was 1:25, and the mixture was homogeneously ground for 30 min with a mortar and pestle. The resulting mixture (0.5 g) was transferred to a ...
Embodiment 3
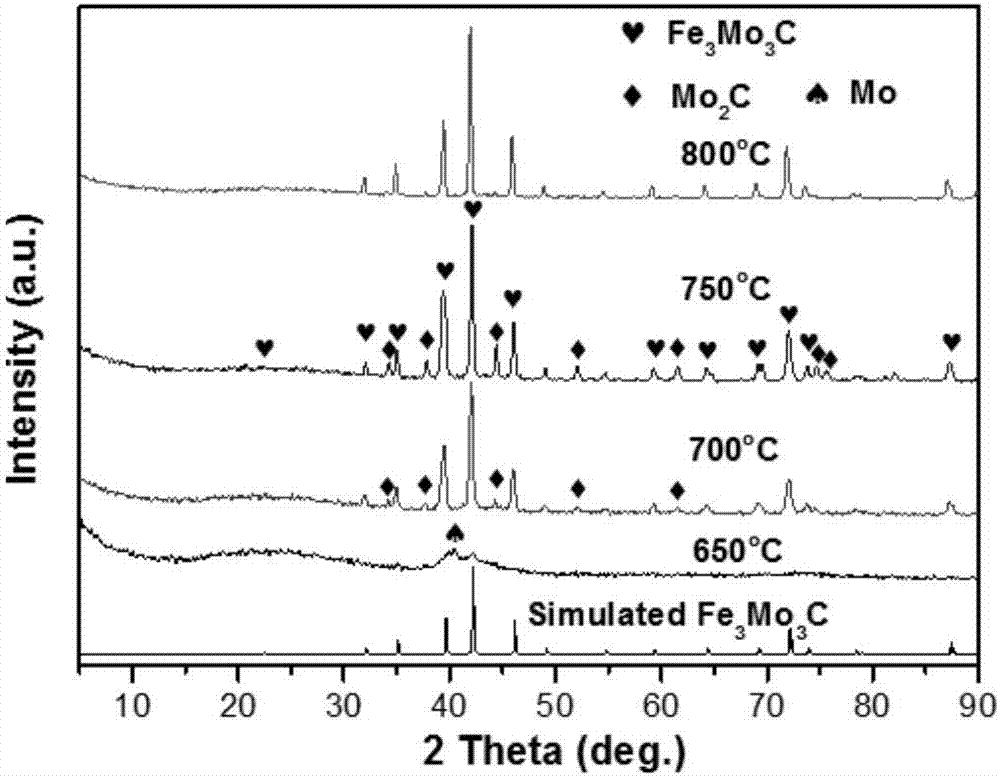
[0030] Embodiment 3: the Na of 0.005mol 2 MoO 4 2H 2 O and 0.005mol FeCl 2 ·6H 2 O was dissolved in 20 mL deionized water, respectively, and stirred to mix at room temperature. A light green precipitate was obtained, and after stirring for 30 minutes, the mixture was transferred and sealed in a 60 mL polytetrafluoroethylene-lined stainless steel autoclave, and kept in an electric oven at 180 °C for 4 hours. Then the autoclave was taken out from the oven and allowed to cool to room temperature naturally. The yellow-green precipitate was collected by centrifugation, washed well with water and ethanol, and dried overnight at 80 °C. Finally, the product was ground into a fine powder for characterization.
[0031] The as-synthesized bimetallic oxide was mixed with activated carbon so that the molar ratio of total metal to carbon was 1:15, and the mixture was uniformly ground for 30 min with a mortar and pestle. The resulting mixture (0.5 g) was transferred to a quartz boat p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com