Process for preparing battery-grade high-purity nano iron phosphate
A nano-iron phosphate, battery-level technology, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problems of ultra-fine iron phosphate that is not easy to wash off, high-end recycling obstacles, and the purity of iron phosphate Low cost, fine particle size, and less waste water discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
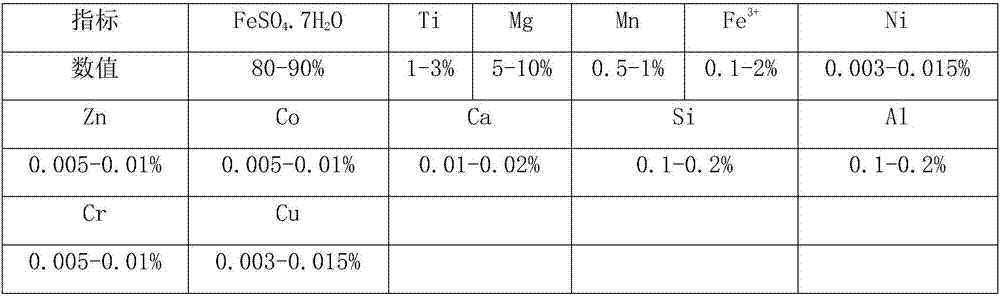
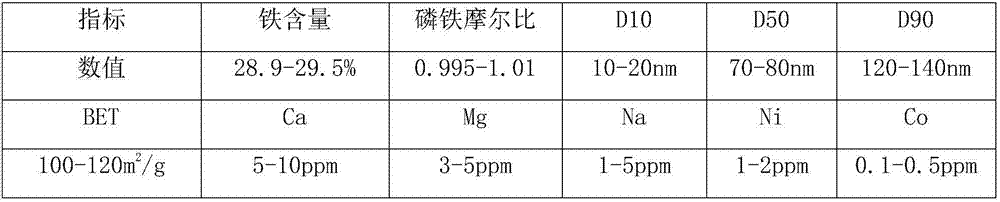
[0052] (1) Add water to dissolve the titanium dioxide by-product until the total iron content in the solution is 45.5g / l, add the mixture of iron hydroxide mud and iron powder, react at 60°C, adjust the final pH of the solution to 5.2, here React at pH for 0.6 hours, filter to obtain the first filter residue and the first filtrate;
[0053] (2) the first filtrate is added acid to adjust the pH to be 1.25, then pass through air oxidation, and the pH of acid maintenance solution is 1.25 simultaneously, to the [Fe in the solution 2+ ] at 3.5g / l, then continue to stand at this pH for 1 hour, filter to obtain the second filter residue and the second filtrate;
[0054] (3) Add acid to the second filtrate, adjust the pH of the solution to be 0.12, then add gac powder, adopt a nanofiltration device to carry out depth filtration, obtain the third filtrate, and di(2-ethylhexyl) phosphoric acid with phosphoric acid and sulfuric acid Mix acid for washing, wash with pure water after washi...
Embodiment 2
[0067] (1) Add water to dissolve the titanium dioxide by-product until the total iron content in the solution is 45.5g / l, add the mixture of ferric hydroxide mud and iron powder, react at 60°C, adjust the final pH of the solution to 5.4, here React at pH for 0.6 hours, filter to obtain the first filter residue and the first filtrate;
[0068] (2) the first filtrate is added acid to adjust the pH to be 1.25, then pass through air oxidation, and the pH of acid maintenance solution is 1.25 simultaneously, to the [Fe in the solution 2+ ] at 3.5g / l, then continue to stand at this pH for 1 hour, filter to obtain the second filter residue and the second filtrate;
[0069] (3) Add acid to the second filtrate, adjust the pH of the solution to be 0.12, then add gac powder, adopt a nanofiltration device to carry out depth filtration, obtain the third filtrate, and di(2-ethylhexyl) phosphoric acid with phosphoric acid and sulfuric acid Mix acid for washing, wash with pure water after was...
Embodiment 3
[0082] (1) Add water to dissolve the titanium dioxide by-product until the total iron content in the solution is 45.5g / l, add the mixture of ferric hydroxide mud and iron powder, react at 60°C, adjust the final pH of the solution to 5.4, here React at pH for 0.6 hours, filter to obtain the first filter residue and the first filtrate;
[0083] (2) adding acid to the first filtrate to adjust the pH to be 1.15, then pass through air for oxidation, while the pH of the acid to maintain the solution is 1.25, to [Fe in the solution 2+ ] at 4.0g / l, then continue to stand at this pH for 1 hour, filter to obtain the second filter residue and the second filtrate;
[0084] (3) Add acid to the second filtrate, adjust the pH of the solution to be 0.12, then add gac powder, adopt a nanofiltration device to carry out depth filtration, obtain the third filtrate, and di(2-ethylhexyl) phosphoric acid with phosphoric acid and sulfuric acid Mix acid for washing, wash with pure water after washing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com