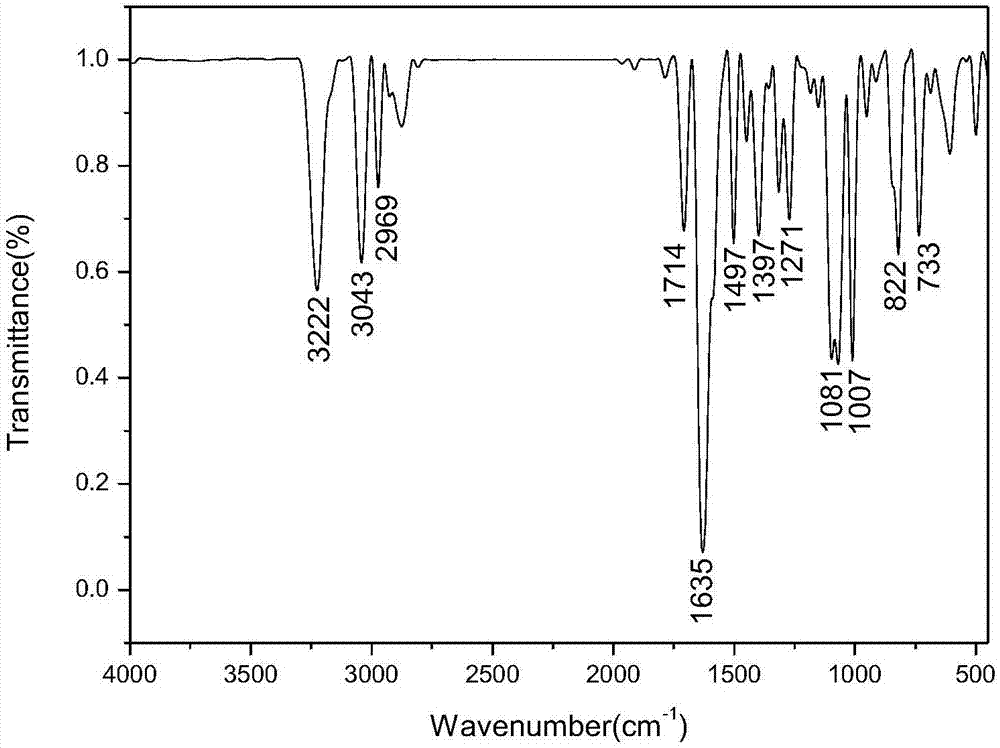
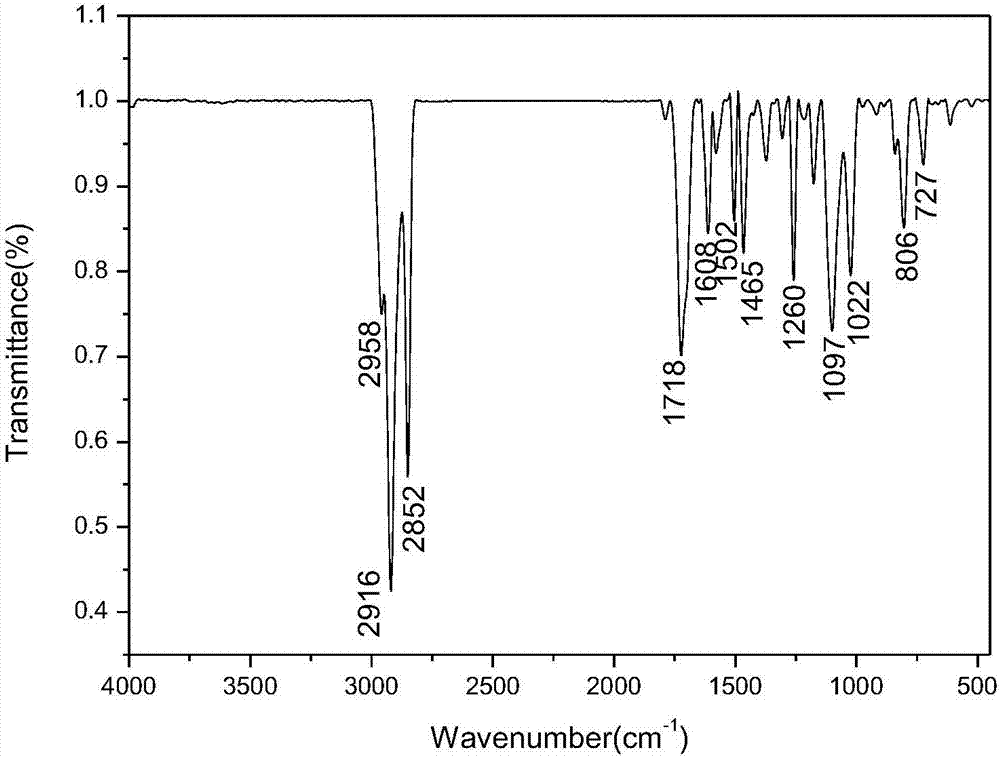
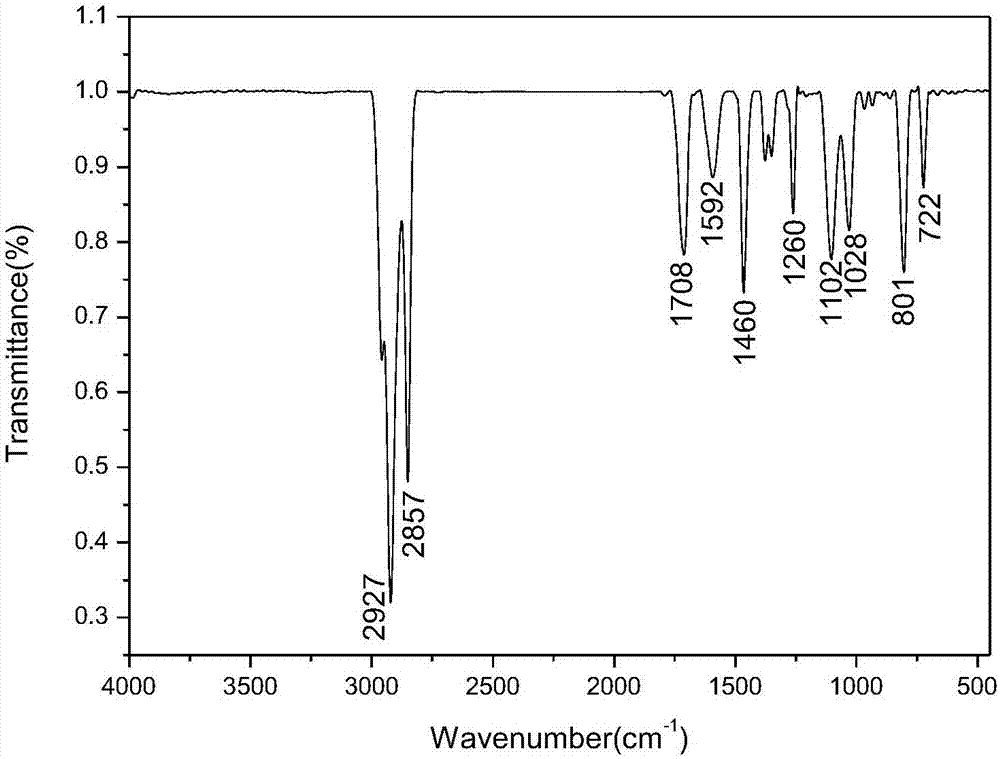
Method for preparing ferrocenyl oxadiazole-based Mannich alkali
A technology based on oxadiazolyl and ferrocene, applied in chemical instruments and methods, metallocene, organic chemistry, etc., can solve the problems of high reaction temperature, long reaction time, and many by-products, etc., and achieves low reaction temperature, The effect of short reaction time and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 0.8mmol of 2-amino-5-(4-methylphenyl)-1,3,4-oxadiazole and 45mmol of formaldehyde to a dry three-necked flask with a reflux condenser at a volume concentration of 37%. Formaldehyde solution, and add 20mL of absolute ethanol as a solvent, add 1.2mmol bismuth nitrate pentahydrate as a catalyst during the stirring process, and use a constant pressure titration funnel to dropwise add acetyl The dehydrated ethanol solution of base ferrocene was reacted at room temperature for 3h, and the reaction process was monitored by TLC plate (when the raw material point of 2-amino-5-(4-methylphenyl)-1,3,4-oxadiazole When it disappears, it means that the reaction is complete. The developer used in the TLC plate is a mixed solution of dichloromethane and methanol with a volume ratio of 10:1). Separation (a mixture of petroleum ether and ethyl acetate with a volume ratio of 3:1 is the first eluent to wash off unreacted acetyl ferrocene, and a mixture of petroleum ether and ethyl aceta...
Embodiment 2
[0034]Add 0.75 mmol of 2-amino-5-(4-methoxyphenyl)-1,3,4-oxadiazole and 45 mmol of formaldehyde to a dry three-necked flask with a reflux condenser to a volume concentration of 37 % formaldehyde solution, and add 20mL of absolute ethanol as a solvent, add 1.2mmol bismuth nitrate pentahydrate as a catalyst during the stirring process, and use a constant pressure titration funnel to add dropwise The dehydrated ethanol solution of acetylferrocene was reacted at room temperature for 5h, and the reaction progress was monitored by a TLC plate (when 2-amino-5-(4-methoxyphenyl)-1,3,4-oxadiazole When the raw material point disappears, it means that the reaction is complete. The developing agent used in the TLC plate is a mixed solution of dichloromethane and methanol with a volume ratio of 10:1). Column chromatographic separation (with a volume ratio of 3:1 the mixed solution of petroleum ether and ethyl acetate as the first eluent to wash off unreacted acetyl ferrocene, with a volume ...
Embodiment 3
[0037] Add 0.7mmol of 2-amino-5-(4-bromophenyl)-1,3,4-oxadiazole and 37% oxadiazole containing 45mmol formaldehyde to a dry three-necked flask with a reflux condenser. Formaldehyde solution, and add 20mL of absolute ethanol as a solvent, add 1.1mmol bismuth nitrate pentahydrate as a catalyst during the stirring process, and use a constant pressure titration funnel to dropwise add acetyl ferrocene containing 1mmol with a concentration of 0.4g / mL The dehydrated ethanol solution of ferrocene was reacted at room temperature for 6h, and the reaction process was monitored by TLC plate (when the raw material point of 2-amino-5-(4-bromophenyl)-1,3,4-oxadiazole disappeared Indicates that the reaction is complete, the TLC plate used developing agent is a mixed solution of dichloromethane and methanol in a volume ratio of 10:1), after the reaction, the solvent absolute ethanol is evaporated by rotary evaporation under reduced pressure, and then separated by column chromatography ( The mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com