Preparation method of lactide and caprolactone random copolymer
A technology of random copolymer and caprolactone, which is used in chemical instruments and methods, compounds containing elements of Group 3/13 of the periodic table, organic chemistry, etc. Copolymer microstructure is uncontrollable and other problems, to achieve the effect of stable properties, controllable microstructure, and excellent random copolymerization performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
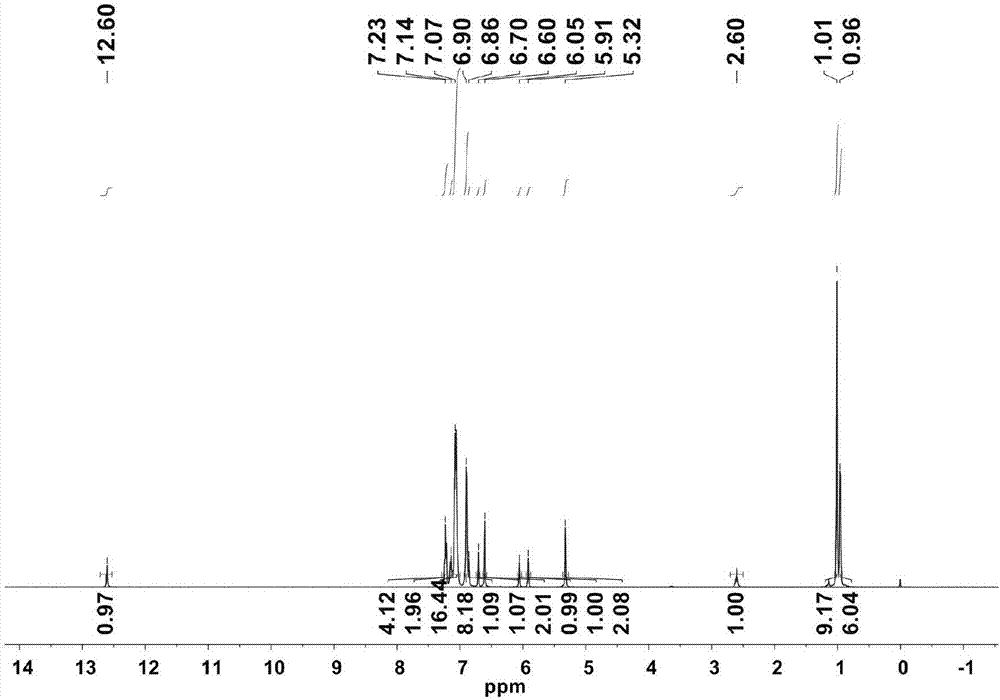
[0044] Bidentate nitroxide ligand 2-Ph 2 CH-4- t Bu-6-(2,6-Ph 2 CH-4- i Pr-C 6 h 2 -N=CH)C 6 h 2 Preparation of OH.
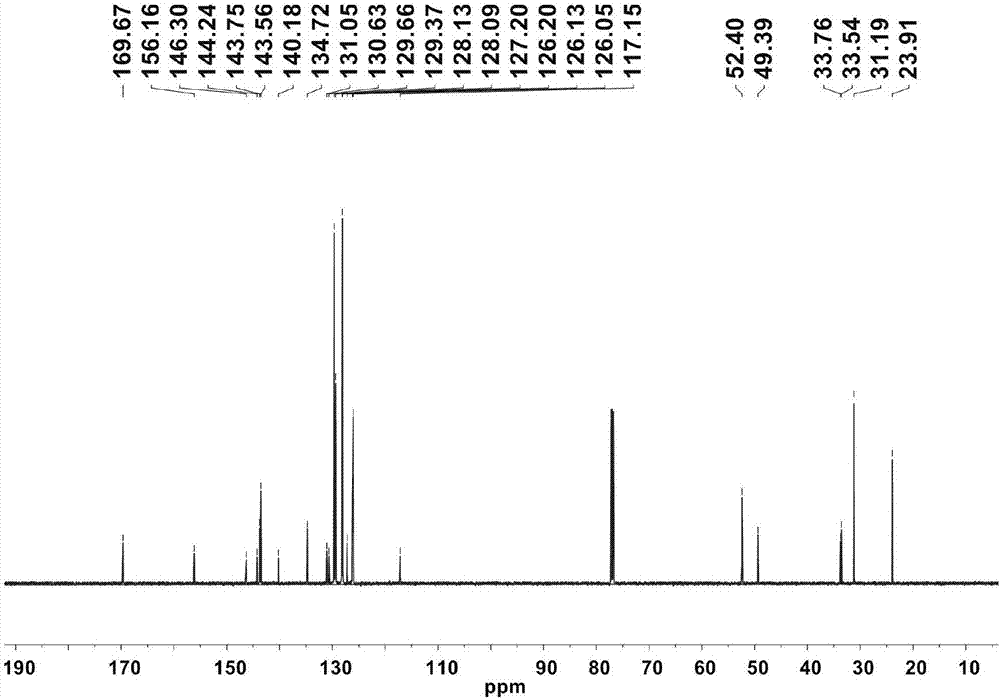
[0045] 2,6-diphenylmethyl-4-isopropylaniline (4.67g, 10.0mmol) and 3-diphenylmethyl-5-tert-butyl salicylaldehyde (3.44g, 10.0mmol) 1:1 After the molar ratio is mixed, add 20mg p-toluenesulfonic acid, react in toluene for 10-18 hours, the reaction temperature is 120°C, then remove the solvent under reduced pressure, add 50mL petroleum ether, and filter to obtain the salicylaldimine ligand (6.75g, 8.5 mmol, 85%). 1 H NMR (CDCl 3 ):δ12.60(s,1H,OH),7.23(t,J=7.5Hz,4H),7.14(t,J=7.5Hz,2H),7.13-7.05(m,16H),6.90(d, J=6.8Hz,8H),6.86(s,1H),6.70(s,1H),6.60(s,2H),6.05(s,1H),5.91(s,1H),5.32(s,2H), 2.60(sept,J=6.7Hz,1H,CH(CH 3 ) 2 ),1.01(s,9H, t Bu),0.96(d,J=6.7Hz,6H,CH(CH 3 ) 2 ). 13 C NMR (CDCl 3 ):δ169.67,156.16,146.30,144.24,143.75,143.56,140.18,134.72,131.05,130.63,129.66,129.37,128.13,128.09,127.20,126.20,126.13,126.05,117.15,52.40,49.39,33.76,33.54,31...
Embodiment 2
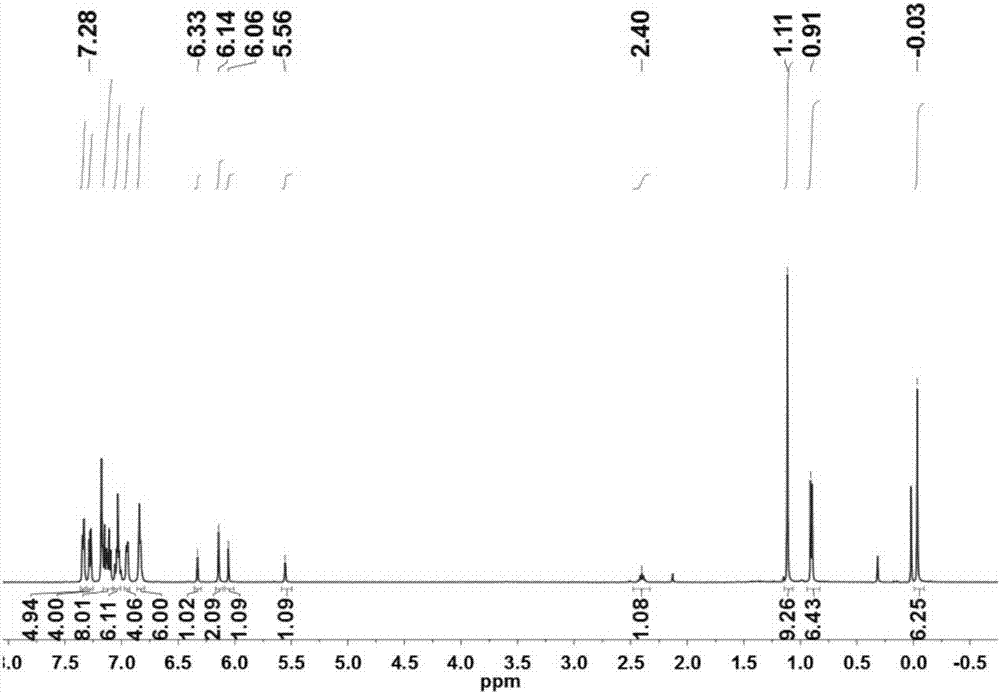
[0047] Bidentate Nitrogen-Oxygen Coordination Aluminum Compound 2-Ph 2 CH-4- t Bu-6-(2,6-Ph 2 CH-4- i Pr-C 6 h 2 -N=CH)C 6 h 2 OAlMe 2 preparation.
[0048] The ligand 2-Ph in Example 1 2 CH-4- t Bu-6-(2,6-Ph 2 CH-4- i Pr-C 6 h 2 -N=CH)C 6 h 2 OH (0.794g, 1.00mmol) was dissolved in 30mL of toluene, 1.0 equivalent of trimethylaluminum was added, stirred at room temperature under nitrogen protection for 12 hours, the solvent was removed under reduced pressure, and washed three times with n-hexane to obtain the corresponding nitrogen-oxygen bidentate complex Aluminum compound (0.807 g, 0.95 mmol, 95%). 1 H NMR (CDCl 3 ):δ7.35-7.33(m,5H),7.28(d,J=7.5Hz,4H),7.15-7.10(m,8H),7.06-7.01(m,6H),6.96-6.91(m,4H ),6.88-6.81(m,6H),6.33(s,1H),6.14(s,2H),6.06(s,1H),5.56(s,1H),2.40(sept,J=6.9Hz,1H) ,1.11(s,9H),0.91(d,J=6.9Hz,6H),-0.03(s,6H,Al-Me). 13 C NMR (CDCl 3 ):δ176.73,160.09,147.13,144.15,143.11,142.78,142.18,138.88,138.21,135.32,134.95,129.95,129.82,129.55,128.41,128...
Embodiment 3
[0050] Compound Al1 and benzyl alcohol catalyze the copolymerization of lactide and caprolactone.
[0051] In the Schlenk bottle, under anhydrous and oxygen-free conditions, add 0.288g lactide and 0.228gε-caprolactone, and add 3mL toluene. 20 μmol of compound Al1 (7.3 mg), 2.1 μL of benzyl alcohol (20 μmol) dissolved in 2 mL of toluene, were added into a Schlenk bottle with a syringe to initiate polymerization. Control the reaction temperature at 110°C for 1 h, add 5 mL of 5% acetic acid methanol solution, pour into methanol to precipitate the polymer, filter and vacuum dry for 24 hours to obtain a copolymer. Conversion: lactide 51% and caprolactone 35%. The number average molecular weight M of the copolymer n :1.09×10 4 g / mol, molecular weight distribution PDI=1.14; average chain length L of lactide and caprolactone monomers LA and L CL 3.1 and 1.4 respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com